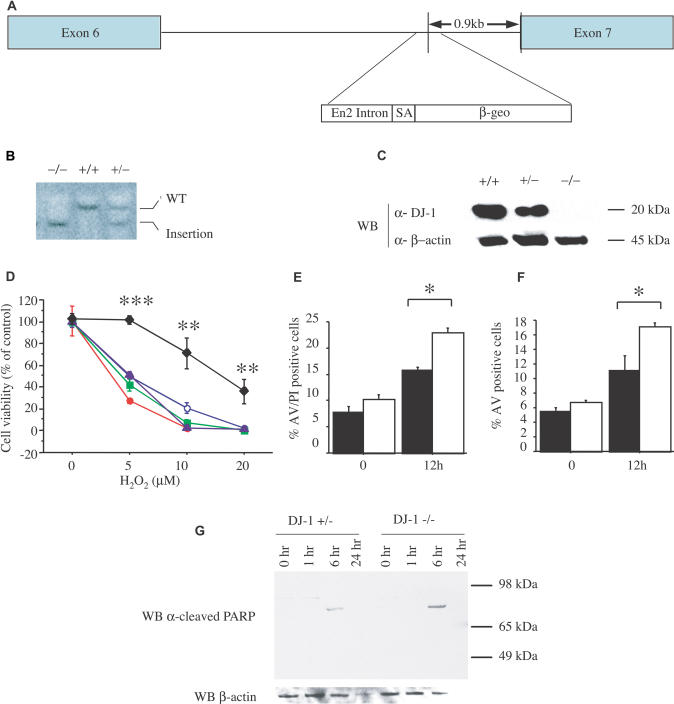
Figure 1. DJ-1-Deficient ES Cells Are Sensitized to Oxidative Stress.
(A) Schematic map of the murine DJ-1 gene in clone F063A04. The retroviral insertion places the engrailed-2 (En2) intron, the splice acceptor (SA), and the β-galactosidase/neomycin resistance gene fusion (β-geo) between exons 6 and 7.
(B) Southern blot analysis of KpnI-digested genomic DNA from DJ-1 homozygous mutant (–/–), WT (+/+), and heterozygous (+/–)cells, probed with murine DJ-1 cDNA. WT DNA shows a predicted 14-kb band (WT), whereas the mutant allele migrates as a 9-kb band (insertion).
(C) Western blot (WB) of ES cell lysates from WT (+/+), DJ-1 heterozygous (+/–), and mutant homozygous (–/–) clones with antibodies to murine DJ-1 (α-DJ-1) or β-actin (α-β-actin). DJ-1 migrates at 20 kDa, β-actin at 45 kDa.
(D) ES cells were exposed to 0, 5, 10, and 20 μM H2O2 for 15 h and viability was assayed by MTT. Responses of DJ-1 heterozygous cells (diamonds) and DJ-1 knockout clones 9 (open circles), 16 (solid circles), 23 (squares), and 32 (triangles) are shown. ** p ≤ 0.01; *** p ≤ 0.0001.
(E and F) Cell death of DJ-1 heterozygous and DJ-1-deficient cells (clone 32) after exposure to H2O2 (10 μM) was quantified by staining with PI and an antibody to AV with subsequent FACS analysis. AV staining marks cells undergoing apoptosis, whereas PI staining indicates dead cells. * p ≤ 0.05.
(G) DJ-1 heterozygous (+/–) and knockout (clone 32; –/–) cells were assayed at 1, 6, and 24 h after treatment with 10 μM H2O2 by Western blotting for cleaved PARP (89 kDa), which indicates apoptosis. No band is seen for cleaved PARP or β-actin for the DJ-1-deficient cells at 24 h due to cell death. Data represent means ± standard error of the mean (SEM) and were analyzed by ANOVA with Fisher's post-hoc test.