Figure 1.
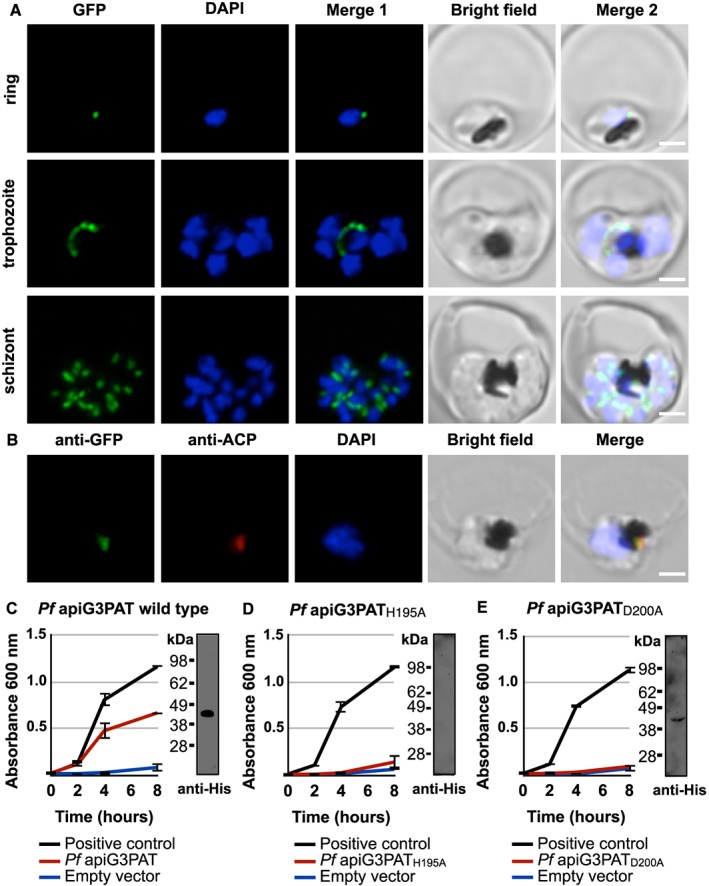
Pf apiG3PAT has a functional apicoplast targeting sequence and rescues growth in a G3PAT‐deficient mutant strain of E. coli. A. Live fluorescence microscopy of Pf apiG3PAT1–70 gfp parasites shows the predicted Pf apiG3PAT apicoplast targeting sequence directs GFP to a discrete cellular compartment in rings, trophozoites and schizonts. B. Immunofluorescence microscopy of Pf apiG3PAT1–70 gfp parasites using antibodies against GFP and the apicoplast marker ACP demonstrates the predicted Pf apiG3PAT apicoplast targeting sequence targets GFP to the apicoplast. DNA stained with DAPI. Scale 3 µm. C. Pf apiG3PAT partially restores growth in a G3PAT‐deficient mutant of E. coli, demonstrating it is active as a G3PAT. Bacteria were transformed with a vector encoding a His‐tagged version of Pf apiG3PAT, the empty vector or E. coli G3PAT positive control. Western blot of bacterial extracts confirms expression of the tagged Pf apiG3PAT (expected mass of 47 kDa). D–E. Mutation of the conserved histidine or aspartate in the ‘HX4D’ motif of Pf apiG3PAT abolishes its ability to restore growth in the E. coli mutant. Bacteria were transformed with vectors encoding His‐tagged versions of the histidine to alanine mutant (Pf apiG3PATH195A), aspartate to alanine mutant (Pf apiG3PATD200A), empty vector or E. coli G3PAT positive control. Western blot of bacterial extracts confirms expression of Pf apiG3PATD200A, but fails to detect Pf apiG3PATH195A, suggesting the conserved histidine may be required for correct folding or stability of the enzyme.
