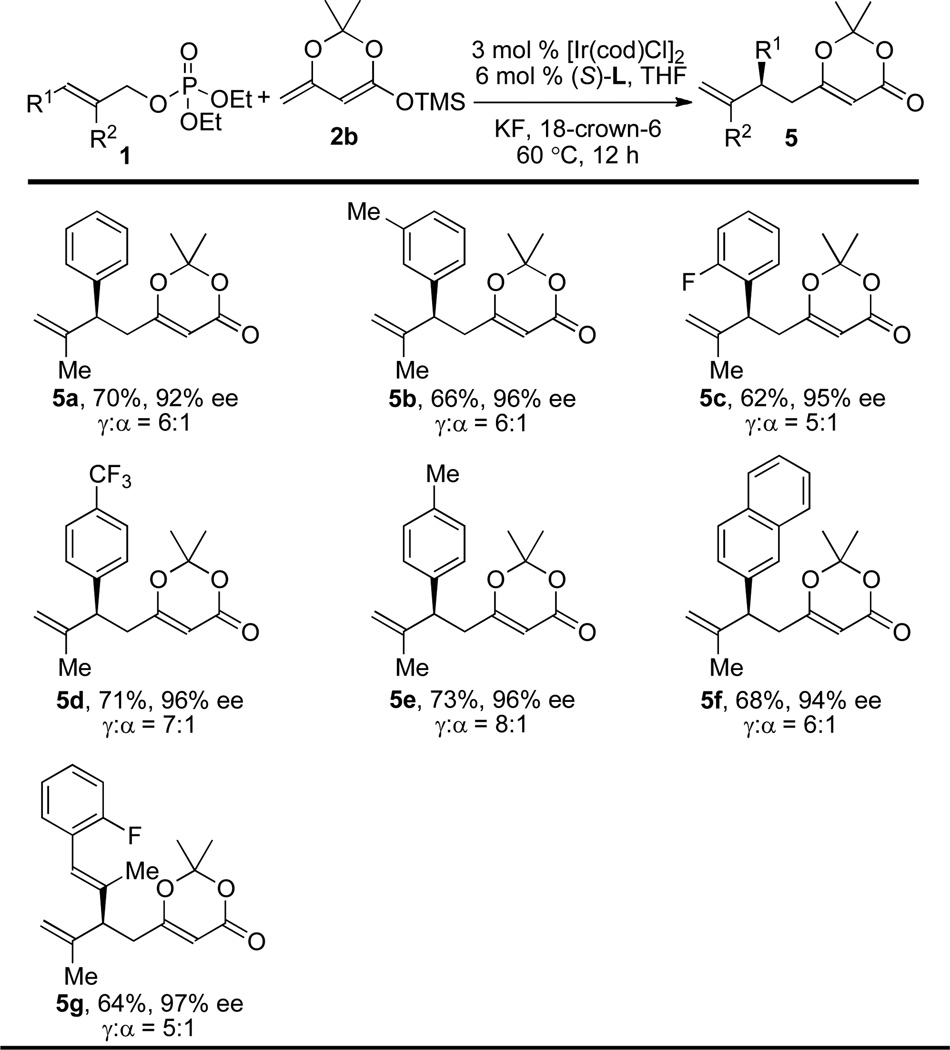
Table 4.
Scope of the Ir-catalyzed Asymmetric Allylic Substitution of Allylic Phosphates 1 with Dinenolate 2b.a–d
Reaction conditions: allylic phosphate 1 (0.2 mmol, 1.0 equiv), dinenolate 2b (0.4 mmol, 2.0 equiv), [Ir(cod)Cl]2 (3 mol %), (Sa,Sc,Sc)-L (6 mol %), KF (1.0 equiv), 18-crown-6 (1.0 equiv), THF (0.4 mL), 60 °C, 12 h.
Ratios of γ- to α-substitution were determined by 1H NMR analysis of the crude reaction mixtures.
Yields of isolated products 5 are listed (the average of at least two runs).
Enantioselectivities were determined by HPLC analysis using a chiral stationary phase.