ABSTRACT
The transport of germ cells from the base of the seminiferous epithelium toward the luminal edge of the tubule lumen in the adluminal compartment during the epithelial cycle is an essential cellular event to support spermatogenesis. Thus, fully developed elongated spermatids (i.e., spermatozoa) can be released at spermiation in late stage VIII in rodents versus late stage II in humans. Earlier studies to examine the molecular mechanism(s) that support germ cell transport, most notably the transport of preleptotene spermatocytes across the blood-testis barrier (BTB), and the transport of elongating spermatids across the adluminal compartment during spermiogenesis, is focused on the adhesion protein complexes at the cell-cell interface. It is generally accepted that cell junctions at the Sertoli cell-cell interface at the BTB, including the actin-based tight junction (TJ), basal ectoplasmic specialization (basal ES, a testis-specific adherens junction) and gap junction (GJ), as well as the intermediate filament-based desmosome undergo constant remodeling to accommodate the transport of preleptotene spermatocytes across the barrier. On the other hand, similar junction dynamics (i.e., disassembly, reassembly and stabilization/maintenance) take place at the Sertoli-spermatid interface. Emerging evidence has shown that junction dynamics at the Sertoli cell-cell vs. Sertoli-germ cell interface are supported by the 2 intriguingly coordinated cytoskeletons, namely the F-actin- and microtubule (MT)-based cytoskeletons. Herein, we provide a brief summary and critically evaluate the recent findings. We also provide an updated hypothetical concept regarding germ cell transport in the testis utilizing the MT-conferred tracks and the MT-specific motor proteins. Furthermore, this cellular event is also supported by the F-actin-based cytoskeleton.
KEYWORDS: actin-based cytoskeleton, blood-testis barrier, ectoplasmic specialization, germ cells, microtubule-based cytoskeleton, seminiferous epithelial cycle, Sertoli cells, spermatogenesis, Testis
Introduction
During spermatogenesis, the best studied cellular events are: (i) self-renewal of undifferentiated spermatogonia via mitosis, (ii) differentiation of spermatogonia to spermatocytes to prepare for meiosis, and meiosis I/II, (iii) differentiation of post-meiotic haploid spermatids through spermiogenesis to spermatozoa, and (iv) the release of spermatozoa into the tubule lumen at spermiation.1-4 Besides these notable events, preleptotene spermatocytes differentiated from type B spermatogonia are transported across the blood-testis barrier (BTB).5-7 Elongating spermatids are also being transported back-and-forth across the adluminal compartment during the epithelial cycle of spermatogenesis before fully developed elongated spermatids (i.e., spermatozoa) which are being lined up at the adluminal edge of the epithelium at state VIII of the cycle before their release into the tubule lumen at spermiation. While this is a notable cellular phenomenon to support spermatogenesis, its biology and the underlying molecular mechanisms remain virtually unknown until recent years. Studies have shown that germ cell transport during the epithelial cycle is supported by the intimate but also intriguing coordination between the microtubule (MT)- and F-actin-based cytoskeletons in Sertoli cells but also at the Sertoli-germ cell interface.8-10 This likely involves MT- and F-actin-specific cytoskeletal regulatory proteins (e.g., nucleation proteins, severing, cross-linking, bundling proteins), motor proteins, and signaling proteins so that the organization of MTs and actin microfilaments can be rapidly altered, modifying from a linear and bundled network, to a branched and unbundled network. These changes, in turn, confer plasticity to the cytoskeletons, making them flexible enough to support the timely and efficient transport of germ cells across the BTB and/or the adluminal compartment during the epithelial cycle. Herein, we briefly review some new findings in the field regarding germ cell transport in the seminiferous epithelium since many of the background information can be found in a recent review from our laboratory.11
Role of microtubule (MT)-based cytoskeleton
Microtubules (MTs) are polarized cylindrical structures composed of protofilaments of α- and ß-tubulin heterodimers via the head-to-tail (i.e., + to - end) addition of α/ß-tubulin dimeric subunits. These α-/ß-dimers are arranged laterally to form a hollow-tube like structure (Fig. 1).12,13 Each α- or ß-tubulin monomer is a ∼450 amino acid-protein with a molecular weight of ∼55 kDa. In short, each MT is a long, straight, polarized and hollow cylindrical structure, composed of 13 laterally associated protofilaments with a lumen having a diameter of ∼17 nm.13 The fast-growing end is the plus (+) end where ß-tubulin is exposed vs. the slow-growing end, the minus (-) end where α-tubulin is exposed (Fig. 1A). Polymerization takes place through interaction of the α-subunit of an incoming dimer with the ß-subunit of a preexisting dimer on a MT protofilament (Fig. 1). On the other hand, γ-tubulin is located at the minus end to constitute a protein complex known as the γ-tubulin ring complex, which is necessary for MT nucleation and is also involved in MT stabilization (Fig. 1A). Studies have shown that MTs serve as tracks, which work in concert with proteins kinases and motor proteins (e.g., dynein, kinesins), for the transport of cell organelles, such as endocytic vesicles, mitochondria, lipids, proteins and others, across cell cytosol in response to changes in cell environment or external cues.14-16 For instance, cytoplasmic dynein complex (CDC) and kinesin-14 family members are the only 2 motor proteins utilizing energy release from hydrolysis of ATP in mammalian cells known to move their cargoes to the minus-end and plus-end directionally, respectively17 (Fig. 1B-E; Fig. 2A-B). Studies of axonal transport (also known as axoplasmic transport, is responsible for the transport of mitochondria, lipids, synaptic vesicles and proteins to and from a neuron cell body through the cytoplasm of its axon) have shown that kinesins mediate MT-dependent anterograde transport (from the neuronal cell body to the axon tip) whereas CDC induces retrograde transport (from the cell periphery to the cell body).14 More important, studies have shown that MT-dependent anterograde (from neuronal cell body to cell periphery in the axon) and retrograde (from cell periphery back to the neuronal cell body) transports are regulated by several protein kinases, which include Akt (PKB), Cdk5, CK2, ERK1/2, GSK-3ß, JNK, p38 MAPK, PINK1, PKA and PKC.14 Many of these protein kinases have been found in the testis, and many of them are associated with the F-actin-rich ultrastructure ectoplasmic specialization (ES).18-20 Some are recently shown to be actively involved in F-actin organization at the basal ES at the Sertoli cell-cell interface, such as Akt1/2,21,22 which was earlier shown to be a putative component of the apical and basal ES23 in the rat testis. In short, the MT-based tracks are analogous to 2-way highways that modulate in-coming and out-going traffic to maintain cellular homeostasis. Perhaps the back-and-forth transport of developing spermatids during spermiation also involves the participation of different protein kinases that dictate the directional transport of spermatids during the epithelial cycle. This possibility must be carefully evaluated in future studies.
Figure 1.
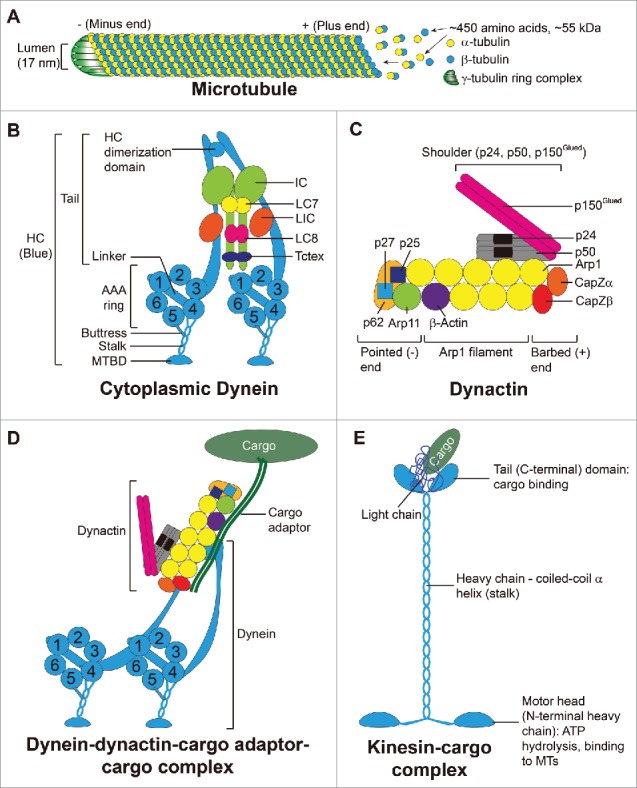
(A) schematic drawing illustrating the organization of microtubule (MT) and the 2 MT-specific motor proteins. (A) Polymerization of a MT takes place through the interaction of the α-tubulin of an incoming dimer with the ß-tubulin of a pre-existing dimer on a MT protofilament at the rapid growth plus (+) end. γ-tubulins form a ring complex at the minus (-) end responsible for nucleation and stabilization of the MT. (B) A functional dynein complex is a dimeric protein. Each monomer is composed of a heavy chain (HC) motor and several subunits: an intermediate chain (IC), a light intermediate chain (LIC), and 3 light chains called LC7 (light chain 7 also called Roadblock, DYNLRB1, or rob1), LC8 (also called DYNLL) and Tctex (also called DYNLT1). Each HC has the N-terminus at the tail and the C-terminal motor unit contains 6 AAA (ATPase Associated with Cellular Activities) domains, AAA1 to 6, and organized into a ring-like structure. The linker, an important mechanical element, connects to AAA1. The coiled-coil stalk extends from AAA4 and leads to the microtubule binding domain (MTBD). The buttress is a coiled-coil hairpin extending from AAA5 to connect to the stalk. (C) Dynactin is also a multi-subunit protein complex composed of a shoulder consisting of p150Glued, p24 and p50 proteins which interacts with the heterodimer CapZα and CapZß at the barbed end, and the dynactin Arp1 filament consisting of 8 Arp1 subunits and one ß-actin, which in turn associate with the pointed end composed of Arp11, p62, p27 and p25 proteins. (D) A simplified model of dynein-dynactin complex in which dynein interacts with dynactin, which in turn interacts with the cargo adaptor to form a functional motor protein to support cargo transport. (E) A schematic drawing illustrating the different domains of a dimeric kinesin motor protein. Each monomer is composed of a heavy chain (HC) and the N-terminal region of the HC contains the motor head which is also the site for ATP hydrolysis and MT binding. This is followed by the coiled-coil α helix that constitutes the stalk, and ends with the tail (C-terminal domain). The two tail domains along with the 2 light chains constitute the cargo binding site to facilitate cargo docking to facilitate cargo (e.g., spermatid, residual body, phagosome) transport. This figure was prepared based on earlier findings as described in text, in particular several recent reports.27,28,69
Figure 2.
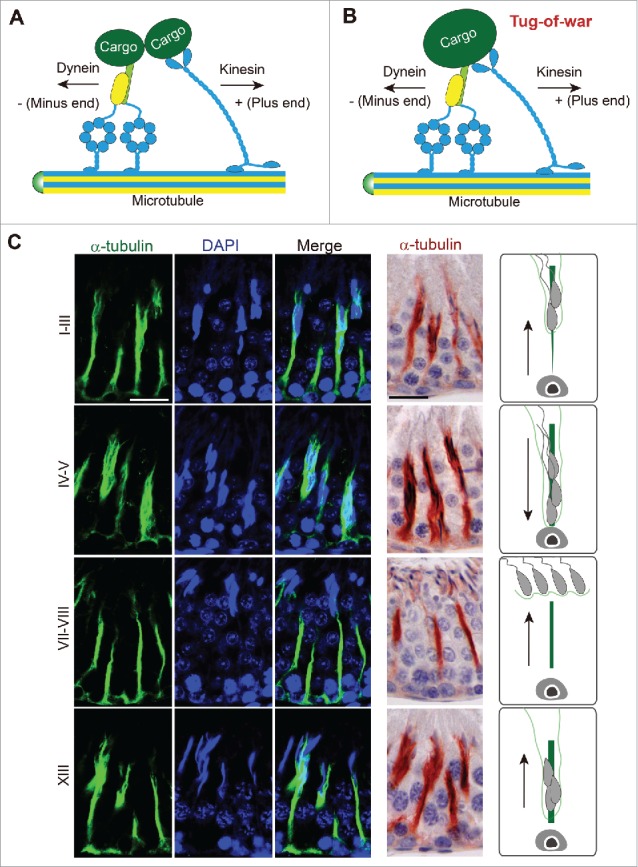
Dynein-dynactin-cargo adaptor complex and microtubule (MT)-based cytoskeleton to support spermatid transport in the testis. (A) The dynein-dynactin-cargo adaptor complex that binds to the MT-conferred track to support cargo transport, including spermatids so that the cargo (i.e., spermatid) can be transported along the MT-based track. The dynein-dynactin-cargo adaptor is responsible to transport cargo to the minus (-) end of the MT track but the kinesin is responsible to transport cargo to the plus (+) end of the MT track. As such, cargo (e.g., spermatid) can be transported across the seminiferous epithelium during the epithelial cycle depending on the polarity of the MT-based track vs. different motor proteins. (B) It is noted that the dynein-dynactin-adaptor complex and the kinesin can compete for the same cargo in a tug-of-war. The eventual winner to move the cargo either to the – or + end of the MT-track is determined by protein kinases, phosphatases and/or Rab GTPases associated with the motor protein which modify the activity of a motor protein through changes in the adaptor proteins or other microtubule-associated proteins (MAPs, e.g., +TIP or –TIP proteins) at the site. (C) A study by fluorescence microscopy that illustrates the presence of track-like structures conferred by MTs, as noted by α-tubulin (green fluorescence), the building block of MTs, in the cross sections of the testis in tubules at different stages of the epithelial cycle (left 3 panels). Scale bar, 60 µm, which applies to all other micrographs in this panel. α-tubulin is also visualized by immunohistochemistry in which MTs appear as reddish-brown precipitates (fourth column from the left). Scale bar, 60 µm, which applies to other micrographs in this panel. The last column is a schematic drawing illustrating the directional transport of spermatids in the specified stages. This experiment was performed using the reagents and antibodies reported earlier from our laboratory.8
Motor proteins and MT-based cytoskeleton to support spermatid transport - the dynein-dynactin-MT complex
It is generally accepted by investigators that MTs serve as the tracks for cellular transports in the seminiferous epithelium, in particular spermatids during spermiogenesis. Studies in mammalian cells have shown transport of cargos along the MT-based tracks require dyneins, which are a large family of motor proteins that utilize the energy derived from ATP hydrolysis to move cargos along MTs in the minus end direction (Fig. 1B-D; Fig. 2A). Cytoplasmic dynein-1 (i.e., dynein) is responsible for MT-minus-end-directed transports of cellular organelles including proteins, mRNA complexes, endosomes, mitochondria and cell nuclei,17,24,25 and it is likely applicable to the minus-end-directed transport of spermatids along MT during spermiogenesis. A schematic drawing of the cytoplasmic dynein is shown in Fig. 1B. The dynein complex is composed of 2 dynein heavy chain motors (HC, 500 kDa each) and other subunits. Each HC also binds to a light intermediate chain (LIC, 60 kDa), an intermediate chain (IC, 70 kDa), and 3 light chains (LCs) of LC7, LC8 and Tctex 1. Each heavy chain (HC) contains the N-terminal tail essential to provide intra-dynein subunit interactions, and a C-terminal motor unit with 6 AAA (ATPase associated with cellular activities) domains (1–6) organized into a ring-like structure with the linker that connects to AAA1 (Fig. 1B). A coiled-coil stalk extends from AAA4, leading to the MT-binding domain (MTBD), and the buttress is a coiled-coil hairpin extends from AAA5 to make contact with the stalk (Fig. 1B). AAA1 is the major site of ATP hydrolysis with other AAA sites involved in regulatory roles. Dynein, in turn, interacts with its cofactor called dynactin to form the functional dynein-dynactin complex, in which dynein IC binds to the p150Glued dimer of dynactin26 (Fig. 1C, D). Recent structural analysis,27,28 have shown that dynactin is composed of several subunits. The most notable subunit is the p150Glued dimer with a microtubule binding domain (MTB) at its N-terminus (Fig. 1C, D). Its C-terminus interacts with the p50/p24 subunits, which in turn interact with the Arp filament. The Arp filament contains a long strand of 5 subunits of Arp1, and a short strand of 3 subunits of Arp1, with the protomer at the pointed end of the short strand being actin. The CapZ heterodimer caps the Arp filament at the barbed end, and the pointed-end complex (Arp11, p62, p27 and p25) caps the filament at the pointed end (Fig. 1C). Together, these component subunits of dynactin create the tail domain that binds cargo (e.g., phagosomes, endosomes, residual bodies), and the motor protein dynein utilizes energy generated via ATP hydrolysis to propel cargo transport as illustrated schematically in Fig. 2A-B. In this context, it is noted that dynein can also bind to cargo directly without the involvement of dynactin through its intermediate chain or light chain. Studies by immunofluorescence analysis and immunohistochemistry (IHC) have demonstrated the distinctive presence of stalk-like structures conferred by MTs (see green fluorescence of α-tubulin or reddish immunoprecipitate of α-tubulin by IHC) which lay longitudinally across the seminiferous epithelium at different stages of the epithelial cycle of spermatogenesis to support spermatid and other cargo (e.g., residual bodies, phagosomes, endocytic vesicles) transport (Fig. 2C). While much research is needed in the coming decade to unravel the precise mechanism(s) by which spermatids are being transported across the seminiferous epithelium, it is likely that the cargo binding domain of the dynein-dynactin complex interacts with a spermatid, making it analogous to a cargo being transported, likely supported also by the F-actin-based cytoskeleton (Fig. 3). As such, spermatids can be propelled toward the minus-end of MT-based tracks (Fig. 4). It is noted that these cellular events are also supported by the spatial expression of +TIP proteins (e.g., EB1, XMAP215)29 vs. -TIP proteins (e.g., CAMSAP1 (calmodulin-regulated spectrin-associated protein 1), CAMSP2, Patronin and Nezha (also known as CAMSAP3)30, which are being used to maintain stability and/or dynamics (i.e., assembly, disassembly and stabilization) of MTs. This thus confers MT plasticity to support cellular functions31, including spermatogenesis in the testis.
Figure 3.
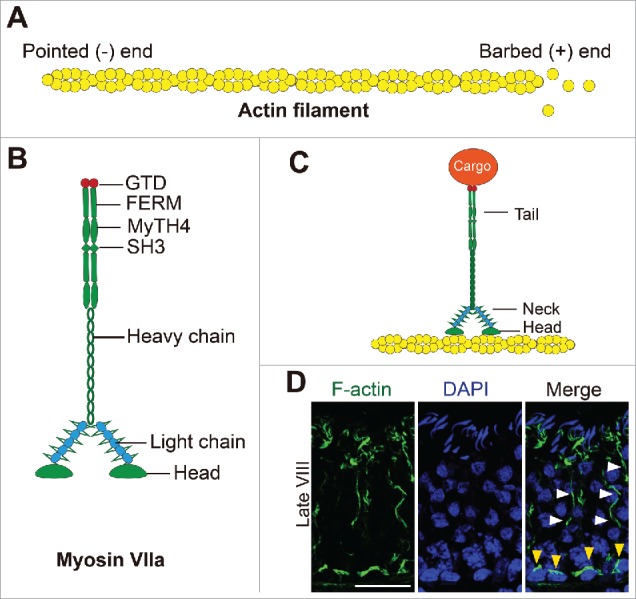
Myosin VIIa and the F-actin-based cytoskeleton that support cargo transport in the testis. (A) Schematic drawing illustrating the organization of an actin microfilament (F-actin) through polymerization G-actin subunits. Microfilaments are then bundled to form the network of actin filament bundles that constitute the ectoplasmic specialization in the testis, which also serve as the attachment site for cell adhesion protein complexes (e.g., N-cadherin-ß-catenin, occludin-ZO-1, nectin-afadin) and to support the transport of spermatids or organelles (i.e., cargoes) by serving as the track. (B) The functional domains of myosin VIIa, a dimeric motor protein associated with F-actin specifically are shown herein. Each polypeptide chain can be divided into the N-terminal head region which also contains the motor domain and the actin-binding site, and the C-terminal tail region contains the FERM (F, 4.1 protein; E, ezrin; R, radixin; M, moesin), MyTH4 (myosin tail homology 4) and SH3 (SRC homology 3) domains and the globular tail domain (GTD) at the C-terminus.51 GTD in the tail domain of myosin recognizes different cargos through direct interactions or mediated by adaptor proteins, such as vezatin in the testis. GTD, supported by the FERM, MyTH4 and SH3 domains, provides the docking site for different cargoes (e.g., spermatids, residual bodies, phagosomes). (C) A simplified model on the support of cargo transport by myosin VIIa in the testis using the F-actin filaments as tracks. (D) Localization of F-actin (green fluorescence, visualized by FITC-phalloidin as earlier described8) in the cross-section of an adult rat testis of a stage VIII tubule, when step 19 spermatids have lined up at the tubule lumen to prepare for their release at spermiation when F-actin expression at the Sertoli cell-step 19 spermatid interface begins to diminish. F-actin is notably detected at the BTB (annotated by yellow arrowheads) near the basement membrane. Also, noted are the track-like structures conferred by F-actin that lay longitudinally across the seminiferous epithelium (annotated by white arrowheads). Scale bar, 60 µm, which applies to other micrographs in this panel.
Figure 4.
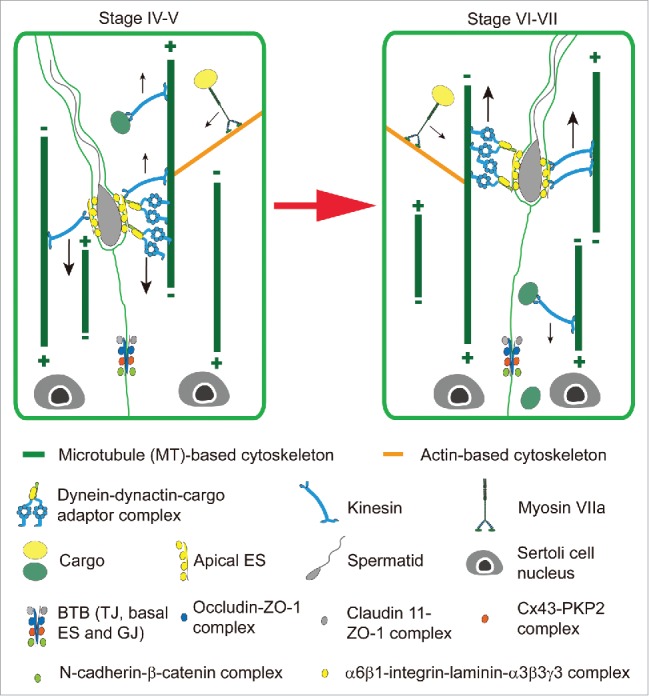
Hypothetical model illustrating the transport of spermatids and other cargoes across the seminiferous epithelium during spermatogenesis is supported by motor proteins along the MT- and the actin-based tracks during spermatogenesis. On the left panel is a stage IV-V tubule when an elongating spermatid is being transported down to the basal compartment assisted by the dynein-dynactin-cargo adaptor complex, as well as other cargoes (e.g., residual bodies, phagosomes, endosomes) through the interactions between motor proteins and the track-like structures provided by the MT- and the actin-based cytoskeletons. It is believed that Sertoli cell MTs are nucleated in the apical cytoplasm and that their plus ends extend towards the base of the SC. However, there is a possibility that the apically nucleated MTs bundles may also have a small population of MTs in which the plus ends extend towards the apex of cell, allowing for directional transport of spermatids towards the base of the epithelium in stage V by the dynein complex, which moves towards the minus end. Preliminary data suggest that dynein expression is elevated at stage V, when spermatids move down to the base of the Sertoli cell, which may provide evidence for the existence of MTs nucleated in the apical cytoplasm, but towards the base of the cell. The right panel depicts the spermatid being transported up to the apex of the SC by the dynein complex along the MT track towards the minus end during stage VI-VII. Again, the small population of MTs with the plus ends extend towards the Sertoli cell apex in the seminiferous epithelium can also be involved in a tug-of-war (see Figure 2) to support spermatid transport across the epithelium (see both panels), and such possibility will require additional investigations in the years to come. Furthermore, the use of different motor proteins, such as dynein (directed a cargo to the minus-end of a MT-based track) and kinesin (directed a cargo to the plus-end of a MT-based track) can also carry the spermatid either to the basal or the apical compartment of the epithelium. The relative localization of apical ES at the Sertoli cell-elongating/elongated spermatid interface and the BTB at the Sertoli cell-cell interface are shown. The apical ES is constituted by apical ES-specific protein complexes (e.g., α6ß1-integrin-laminin-α3ß3γ 3 complex, nectin 2/3-afadin complex), whereas the BTB is composed of TJ- (e.g, occludin-ZO-1, claudin 11-ZO-1), basal ES- (e.g., N-cadherin-ß-catenin) and GJ- (e.g., Cx43-PKP2) proteins. Abbreviations; Cx43, connexin 43; BTB, blood-testis barrier; ES, ectoplasmic specialization; GJ, gap junction; PKP2, plakophilin-2; TJ, tight junction; ZO-1, zonula occludens 1.
Furthermore, besides dynein, kinesin is another family of MT-based motor proteins (> 40 kinesin proteins in humans and organized into at least 14 families), responsible for MT-plus-end-directed transports of cell organelles32,33 (Fig. 1E). Kinesin usually assembles as a homodimeric or heterotrimeric protein and schematically shown in Fig. 1E. In short, kinesin consists of N-terminal motor domains with ATPase activity and the MT binding site that moves to the plus-end of MTs, linked by a coiled-coil rod (α helix stalk) to a cargo binding tail at the C-terminus where accessory subunits of KAPs or KLCs are found.34 Taken collectively, developing spermatids can either move up or down the seminiferous epithelium at different stages of the epithelial cycle, perhaps by recruiting either plus-end (i.e., kinesin) or minus-end (i.e., dynein) directed motor proteins (Fig. 4). In this context, it is of interest to note that plus-end-directed kinesin motors and minus-end-directed dynein motors are sometimes simultaneously attached to the same cargo, engaging in a stochastic ‘tug-of-war” (Fig. 2B). The eventual winning motor protein that dictates the directional transport of a cargo is determined by the presence of protein kinases, phosphatases and/or Rab GTPases associated with the motor protein, which can modify the activity of a motor protein through changes in the adaptor proteins or other microtubule-associated proteins (MAPs). Also involved is the polarized F-actin microfilaments which also serve as the tracks to coordinately support cellular transport such as serving as a guide for MT growth31,33,35 (Figs. 3 and 4). The net result of these changes thus favor either plus-end or minus-end directed motor-mediated directional cargo transport.
Role of motor protein myosin VIIa and F-actin-based cytoskeleton to support spermatid transport
Similar to other epithelia, many of the occluding (e.g., TJ), anchoring (e.g., basal ES) and communicating (e.g., GJ) junctions in the testis, found at the Sertoli cell-cell interface that constitute the BTB, and the apical ES at the Sertoli-spermatid (step 8–19) interface all utilize F (filamentous)-actin for attachment (Fig. 3). Each actin microfilament is composed of polymerized G (globular)-actin, and F-actin is also a polarized ultrastructure, similar to a MT filament, containing a fast growing barbed (+) end and a slow growing pointed (-) end36-38 (Fig. 3A). In the seminiferous epithelium of the testis, actin microfilaments are usually bundled and associated closely with the ectoplasmic specialization (ES), both at the Sertoli-spermatid interface known as the apical ES and at the Sertoli cell-cell interface called basal ES to support spermatid and Sertoli cell adhesion and spermatid/germ cell transport.39-41 Actin dynamics (i.e., disassembly, reassembly and stabilization) are conferred by actin nucleation proteins (e.g., formin 1), bundling proteins (e.g., palladin, plastin 3, fascin 3), severing/cleavage proteins (e.g., cofilin-1) and unbundling/nucleation protein (e.g., the Arp2/3 complex). Similar to MTs, the transport of cellular organelles (e.g., endocytic vesicles, phagosomes, residual bodies) and spermatids across the F-actin-based tracks also require motor proteins such as myosin VIIa found in the testis to move the cargo (e.g., spermatids) (Fig. 3B, C). As noted in Fig. 3D, F-actin is localized extensively at the BTB near the basement membrane (annotated by yellow arrowheads), and at the apical ES at the Sertoli cell-elongated spermatid interface to support these junction structures. However, F-actin also confers track-like structures (annotated by white arrowheads) that lay longitudinally across the seminiferous epithelium (Fig. 3D), consistent with the concept that these track-like structures are working in concert with the MT-based tracks, in coordination with motor proteins (e.g., myosin VIIa) (Fig. 3B, C) to support transport of cell organelles (e.g., residual bodies, phagosomes) and likely developing spermatids during the epithelial cycle, similar to other mammalian cells and/or epithelia35 (Fig. 4). Similar to MT-specific motor proteins, there are many motor proteins specific to actin-based cytoskeleton. The best studied F-actin-based motor proteins are myosins which are products of at least 40 myosin genes and can be categorized into at least 14 classes.42 Myosin VIIa is a member of the myosin superfamily found in the testis but also expressed in the inner ear, retina, kidney, and lung.43 It is also a common component of cilia and microvilli.44 In the testis, myosin VIIa forms a complex with vezatin45 and it has been postulated to be involved in spermatid transport.46,47 Similar to other myosins, myosin VIIa is a dimeric protein. Each monomer is composed of the distinctive head, neck and tail domains (Fig. 3B-C). The N-terminal head and motor domain interacts with actin through its actin binding site, and capable of hydrolyzing ATP to generate energy to propel the transport of cargos along the actin-based track.48 The neck domain (or lever arm) contains IQ motifs (having the consensus IQXXXRGXXR sequence), and the neck domain transduces and amplifies the force generated by motor domain to support cargo propelling.49,50 The tail domain is the cargo binding site which contains the FERM, SH3 and MyTH4 domains to recruit and provide the docking site for cargos, and also the C-terminal globular tail domain (GTD).51 GTD in the tail domain of myosin recognizes different cargos through direct interactions or is mediated by adaptor proteins, such as vezatin in the testis, which in turn interacts with organelle (e.g., phagosome)-specific Rab GTPase for organelle-specific cargo transport.52
Coordination of MT- and actin-based cytoskeletons to support spermatid transport during the epithelial cycle of spermatogenesis
The track-like structures conferred by MTs and F-actin in the seminiferous epithelium as shown in Fig. 2C and Fig. 3D support the notion that they are important to support spermatid and organelle (e.g., residual bodies, phagosomes – the cargos) transport since these are analogous to “freeways,” with the motor proteins serving as the engines (Fig. 4). It is noted that there are virtually no functional studies in the literature to support this concept, however, findings based on the use of several animal models based on several recent reports support the concept that the MT- and F-actin-based cytoskeletons are involved in spermatid and organelle transport during the epithelial cycle of spermatogenesis.
The adjudin model
A recent report,8 using an animal model based on treatment of rats with adjudin (at 50 mg/kg b.w., by oral gavage) known to induce exfoliation of germ cells (beginning with elongating/elongated spermatids within ∼6 to 9 hr following drug administration, to be followed by round spermatids (in ∼3 days) and spermatocytes (in ∼6.5 days)),53 due to initial disruption of the actin-rich testis-specific apical ES to be followed by desmosome and/or gap junction by this drug,54 has shed light on the role of these cytoskeletons to support spermatid transport. This animal model, similar to other drug models such as treatment of rats with environmental toxicant cadmium or other toxicants (e.g., carbendazim, 2,5-hexanedione, colchicine), is known to induce germ cell exfoliation from the testis.55-57 It was noted that while spermatids near the tubule lumen at stage VI to early VIII were depleting from the epithelium, analogous to the release of sperm at spermiation, many step 19 spermatids remained entrapped deep inside the epithelium.8 These step 19 spermatids were consistently found in the epithelium of stages IX, X, XI and XII tubules, until they were eventually engulfed and degraded by Sertoli cells.8 More striking is the observation that the actin-based apical ES that anchored these spermatids deep inside the epithelium was found to be degenerated wherein the apical ES adhesion proteins (e.g., ß1-integrin, nectin-3) and the underlying F-actin were either downregulated or grossly disorganized or mis-localized.8 These findings suggest that even though apical ES and the underlying F-actin cytoskeletal network that supported step 19 spermatid adhesion had been compromised by adjudin treatment, these spermatids located deep inside the epithelium failed to be transported to the tubule lumen for their release, unlike those spermatids that resided near the tubule lumen which were emptied into the tubule lumen earlier. A careful examination of the seminiferous epithelium from these adjudin-treated rats vs. control testes had shown that the F-actin- and the MT-based track-like structures were virtually non-existent in tubules undergoing premature spermiation in the adjudin treated rats as soon as ∼3–6 hr following treatment.8 These findings thus suggest that the presence of these track-like structures are necessary and essential for the transport of spermatids across the epithelium. As such, even though the only anchoring device, namely the apical ES, that supports step 19 spermatids was compromised, the lack of track-like structures rendered these spermatids incapable of being transported to the tubule lumen, confirming the functional significance of these MT- and F-actin-based tracks for spermatid transport.
The F5-peptide model
Of course, one could argue that while this hypothesis is of interest, interpretation of these observations may have been oversimplified due to the fact that adjudin is a drug not locally produced in the body and could have other yet-to-be identified effects. There are 2 additional lines of evidence based on recent studies to support the notion that these track-like structures are necessary and crucial to support spermatid transport. First, overexpression of an F5-peptide - a biologically active peptide derived from apical ES protein laminin-γ3 during apical ES breakdown to prepare for spermiation and is known to perturb BTB remodeling to coordinate the events of spermiation and BTB restructuring across the seminiferous epithelium at stage VIII of the cycle58 – in the testis was shown to induce F-actin- and MT-based disorganization.59 Similar to findings from the adjudin model,8 virtually all the track-like structures conferred by F-actin and MTs were no longer visible that laid longitudinally across the epithelium in the tubules examined following overexpression of F5-peptide in the rat testis.59 More striking was that many step 19 spermatids remained entrapped deep inside the epithelium in stage IX-X and XI-XIII tubules even though the apical ES and the underlying F-actin network were compromised.59 Collectively, these findings illustrate that even though the apical ES has been compromised, step 19 spermatids failed to be depleted from the epithelium due the lack of track-like structures to support their transport to the edge of the aluminal compartment to be released into the tubule lumen.
The formin 1 knockdown model
A knockdown of formin 1 – an actin nucleation protein known to generate long stretches of actin microfilaments across cell cytosol,60-62 such as in Sertoli cells10 and is also involved in MT dynamics via its MT binding domain63 – by RNAi was also found to grossly perturb the organization of F-actin microfilaments and MTs across the seminiferous epithelium.9 For instance, the track-like structures conferred by actin microfilaments and MTs that laid longitudinally across the epithelium virtually vanished in most of the tubules examined following the knockdown of formin 1.9 Similar to the other 2 models, step 19 spermatids were found entrapped deep inside the epithelium in many stage VIII-XII tubules when the apical ES was grossly disrupted,9 illustrating the lack of the track-like structures analogous to the missing freeways, preventing proper transport of cargos (i.e., spermatids) deep inside the epithelium into the luminal edge (i.e., the port) for their release into the tubule lumen. Collectively, findings from these 3 models conclusively demonstrate the significance of the tracks conferred by F-actin and MTs to support spermatid transport. These findings also support the notion that actin microfilaments and MTs likely function as a combined entity to support spermatid transport collaboratively (Fig. 4). This possibility is not entirely unexpected since MTs and actin microfilaments at the ES are intimately associated based on studies by electron microscopy.15,39 Recent studies suggest that the MT-binding proteins, such as EB1 (end binding protein 1, a +TIP, plus-end tracking protein) may serve as a crucial cross-talker between the F-actin- and MT-based cytoskeletons. For instance, a knockdown of EB1 by RNAi not only perturbs the organization of MTs across the Sertoli cell cytosol, it also impedes the organization of actin microfilaments across the Sertoli cell.64
Concluding remarks and future perspectives
As briefly summarized here, the emerging concept regarding the molecular mechanism(s) that supports the transport of germ cells, mostly notably the transport of preleptotene spermatocytes across the BTB and the transport of developing spermatids across the adluminal compartment during spermatogenesis, involved more than cell adhesion protein complexes at the Sertoli cell-cell or Sertoli-germ cell interface. In fact, the 2 intimately localized F-actin and MT cytoskeletons are working in concert to support germ cell movement. In short, actin microfilaments provide the infrastructure to support cell adhesion proteins, such as occludin-ZO-1 and claudin-ZO-1 at the TJ vs. N-cadherin-ß-catenin and nectin 2-afadin at the basal ES, and α6ß1-integrin-laminin-α3β3γ3 and nectin 3-afadin at the apical ES.40,41 However, F-actin and most notably MTs provide the track-like structures that usually lay longitudinally across the seminiferous epithelium, and which work in concert with the F-actin- or MT-specific motor proteins to support spermatid transport. The directional transport of spermatid across the epithelium, on the other hand, is supported by the polarized actin filaments and MTs, such as through the involvement of different motor proteins that induce directional transport of cargos (e.g., dynein or kinesin moves its cargo to the -end or +end of MTs, respectively). This also provides an efficient system to modulate directional transport of spermatids during the epithelial cycle. Of course, other actin- and MT-binding proteins vs. +TIP and -TIP are also involved in this process so that actin microfilaments and MTs can be rapidly organized into either a bundled or unbundled configuration with different extents of stability to confer plasticity of the actin- or MT-based tracks by directing the transport of spermatids across the epithelium. In this context, it is of interest to note that studies on a small GTPase Rap1 also shed new insightful information regarding the biology of spermatid transport in the testis,65-67 which has recently been eminently reviewed.68 The most important breakthrough in these earlier studies regarding the functional significance of Rap1 in spermatogenesis is the generation of a transgenic mouse model in which spermatids from these mice express an inactive Rap1 mutant.67 These mice are subfertile with considerably reduced sperm counts,67 which is the result of extensive spermatid loss from the epithelium due to an inability of spermatids to anchor onto the Sertoli cells to sustain their development via spermiogenesis.67 In short, the apical ES function is compromised in these Rap1 mutant mice. It is likely that other GTPases (or the Rap1 expressed in Sertoli cells continues to sustain low level of spermiogenesis) supersede the lost function of Rap1 due to its inactivation in spermatids, at least in part, so that these mice remain subfertile. However, this mouse model provides a valuable tool for future studies to examine if Rap1 is involved in vesicle-based intracellular transport to alter the spatial expression of actin- or MT-regulatory proteins, which in turn modulate the function of cytoskeletons to confer spermatid transports and other cellular events pertinent to spermiogenesis. Furthermore, Rap1 expressed by spermatids during spermiogenesis may also be involved in modulating Sertoli cell function through intracellular trafficking events that impede spermatid-Sertoli communication through gap junctions or intercellular bridges. Such possibilities must be carefully evaluated in future studies.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Funding
This work was supported by grants from the National Institutes of Health (NICHD, R01 HD056034 to C.Y.C.; U54 HD029990 Project 5 to C.Y.C.). W.Q. was supported in part by a fellowship from the Economic Development Council, and the Noopolis Foundation.
References
- [1].Ehmcke J, Schlatt S. A revised model for spermatogonial expansion in man: lessons from non-human primates. Reproduction 2006; 132:673-80; PMID:17071768; http://dx.doi.org/ 10.1530/rep.1.01081 [DOI] [PubMed] [Google Scholar]
- [2].Schlatt S, Ehmcke J. Regulation of spermatogenesis: an evolutionary biologist's perspective. Semin Cell Dev Biol 2014; 29:2-16; PMID:24685618; http://dx.doi.org/ 10.1016/j.semcdb.2014.03.007 [DOI] [PubMed] [Google Scholar]
- [3].de Kretser DM, Kerr JB. The cytology of the testis. : Knobil E, Neill JB, Ewing LL, Greenwald GS, Markert CL, Pfaff DW, eds The Physiology of Reproduction Vol 1 New York: Raven Press, 1988:837-932. [Google Scholar]
- [4].Hermo L, Pelletier RM, Cyr DG, Smith CE. Surfing the wave, cycle, life history, and genes/proteins expressed by testicular germ cells. Part 1: background to spermatogenesis, spermatogonia, and spermatocytes. Microsc Res Tech 2010; 73:241-78; PMID:19941293; http://dx.doi.org/ 10.1002/jemt.20783 [DOI] [PubMed] [Google Scholar]
- [5].Hess RA, de Franca LR. Spermatogenesis and cycle of the seminiferous epithelium. Adv Exp Med Biol 2008; 636:1-15; PMID:19856159; http://dx.doi.org/ 10.1007/978-0-387-09597-4_1 [DOI] [PubMed] [Google Scholar]
- [6].Parvinen M. Regulation of the seminiferous epithelium. Endocr Rev 1982; 3:404-17; PMID:6295753; http://dx.doi.org/ 10.1210/edrv-3-4-404 [DOI] [PubMed] [Google Scholar]
- [7].Wang CQF, Cheng CY. A seamless trespass: germ cell migration across the seminiferous epithelium during spermatogenesis. J Cell Biol 2007; 178:549-56; PMID:17698604; http://dx.doi.org/ 10.1083/jcb.200704061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Tang EI, Lee WM, Cheng CY. Coordination of actin- and microtubule-based cytoskeletons supports transport of spermatids and residual bodies/phagosomes during spermatogenesis in the rat testis. Endocrinology 2016; 157:1644-59; PMID:26894662; http://dx.doi.org/ 10.1210/en.2015-1962 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Li N, Mruk DD, Tang EI, Lee WM, Wong CK, Cheng CY. Formin 1 regulates microtubule and F-actin organization to support spermatid transport during spermatogenesis in the rat testis. Endocrinology 2016; 157:2894-908; PMID:27145014; http://dx.doi.org/ 10.1210/en.2016-1133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Li N, Mruk DD, Wong CKC, Han D, Lee WM, Cheng CY. Formin 1 regulates ectoplamic specialization in the rat testis through its actin nucleation and bundling activity. Endocrinology 2015; 156:2969-83; PMID:25901598; http://dx.doi.org/ 10.1210/en.2015-1161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Xiao X, Mruk DD, Wong CKC, Cheng CY. Germ cell transport across the seminiferous epithelium during spermatogenesis. Physiology 2014; 29:286-98; PMID:24985332; http://dx.doi.org/ 10.1152/physiol.00001.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Tang EI, Mruk DD, Lee WM, Cheng CY. Cell-cell interactions, cell polarity, and the blood-testis barrier. : Cell Polarity 1 Ed Ebnet K. Geneva, Springer International Publishing; 2015. 303-326. [Google Scholar]
- [13].Penazzi L, Bakota L, Brandt R. Microtubule dynamics in neuronal development, plasticity, and neurodegeneration. Int Rev Cell Mol Biol 2016; 321:89-169; PMID:26811287; http://dx.doi.org/ 10.1016/bs.ircmb.2015.09.004 [DOI] [PubMed] [Google Scholar]
- [14].Gibbs KL, Greensmith L, Schiavo G. Regulation of axonal transport by protein kinases. Trends Biochem Sci 2015; 40:597-610; PMID:26410600; http://dx.doi.org/ 10.1016/j.tibs.2015.08.003 [DOI] [PubMed] [Google Scholar]
- [15].Tang EI, Mruk DD, Cheng CY. Regulation of microtubule (MT)-based cytoskeleton in the seminiferous epithelium during spermatogenesis. Semin Cell Dev Biol 2016; 59:35-45; PMID:26791048; http://dx.doi.org/25619249 10.1016/j.semcdb.2016.01.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Bachmann A, Straube A. Kinesins in cell migration. Biochem Soc Trans 2015; 43:79-83; PMID:25619249; http://dx.doi.org/ 10.1042/BST20140280 [DOI] [PubMed] [Google Scholar]
- [17].Schmidt H, Carter AP. Review: Structure and mechanism of the dynein motor ATPase. Biopolymers 2016; 105:557-67; PMID:27062277; http://dx.doi.org/ 10.1002/bip.22856 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Lee NPY, Cheng CY. Protein kinases and adherens junction dynamics in the seminiferous epithelium of the rat testis. J Cell Physiol 2005; 202:344-60; PMID:15389520; http://dx.doi.org/ 10.1002/jcp.20119 [DOI] [PubMed] [Google Scholar]
- [19].Lee NPY, Mruk DD, Wong CH, Cheng CY. Regulation of Sertoli-germ cell adherens junction dynamics in the testis via the nitric oxide synthase (NOS)/cGMP/protein kinase G (PRKG)/β-catenin (CATNB) signaling pathway: An in vitro and in vivo study. Biol Reprod 2005; 73:458-71; PMID:15858215; http://dx.doi.org/ 10.1095/biolreprod.105.040766 [DOI] [PubMed] [Google Scholar]
- [20].Siu MKY, Wong CH, Xia W, Mruk DD, Lee WM, Cheng CY. The β1-integrin-p-FAK-p130Cas-DOCK180-RhoA-vinculin is a novel regulatory protein complex at the apical ectoplasmic specialization in adult rat testes. Spermatogenesis 2011; 1:73-86; PMID:21866278; http://dx.doi.org/ 10.4161/spmg.1.1.15452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Mok KW, Chen H, Lee WM, Cheng CY. rpS6 regulates blood-testis barrier dynamics through Arp3-mediated actin microfilament organization in rat Sertoli cells. An in vitro study. Endocrinology 2015; 156:1900-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Mok KW, Mruk DD, Cheng CY. rpS6 regulates blood-testis barrier dynamics through Akt-mediated effects on MMP-9. J Cell Sci 2014; 127:4870-82; PMID:25217631; http://dx.doi.org/ 10.1242/jcs.152231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Siu MKY, Wong CH, Lee WM, Cheng CY. Sertoli-germ cell anchoring junction dynamics in the testis are regulated by an interplay of lipid and protein kinases. J Biol Chem 2005; 280:25029-47; PMID:15870075; http://dx.doi.org/ 10.1074/jbc.M501049200 [DOI] [PubMed] [Google Scholar]
- [24].Carter AP, Diamant AG, Urnavicius L. How dynein and dynactin transport cargos: a structural perspective. Curr Opin Struct Biol 2016; 37:62-70; PMID:26773477; http://dx.doi.org/ 10.1016/j.sbi.2015.12.003 [DOI] [PubMed] [Google Scholar]
- [25].Xiang X, Qiu R, Yao X, HN Arst Jr., Penalva MA, Zhang J. Cytoplasmic dynein and early endosome transport. Cell Mol Life Sci 2015; 72:3267-80; PMID:26001903; http://dx.doi.org/ 10.1007/s00018-015-1926-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Schroer TA. Dynactin. Annu Rev Cell Dev Biol 2004; 20:759-79; http://dx.doi.org/ 10.1146/annurev.cellbio.20.012103.094623 [DOI] [PubMed] [Google Scholar]
- [27].Chowdhury S, Ketcham SA, Schroer TA, Lander GC. Structural organization of the dynein-dynactin complex bound to microtubules. Nat Struct Mol Biol 2015; 22:345-7; PMID:25751425; http://dx.doi.org/ 10.1038/nsmb.2996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Urnavicius L, Zhang K, Diamant AG, Motz C, Schlager MA, Yu M, et al. . The structure of the dynactin complex and its interaction with dynein. Science 2015; 347:1441-6; PMID:25814576; http://dx.doi.org/ 10.1126/science.aaa4080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Akhmanova A, Steinmetz MO. Microtubule +TIPs at a glance. J Cell Sci 2010; 123:3415-9; PMID:20930136; http://dx.doi.org/ 10.1242/jcs.062414 [DOI] [PubMed] [Google Scholar]
- [30].Akhmanova A, Hoogenraad CC. Microtubule minus-end-targeting proteins. Curr Biol 2015; 25:R162-71; PMID:25689915; http://dx.doi.org/ 10.1016/j.cub.2014.12.027 [DOI] [PubMed] [Google Scholar]
- [31].Akhmanova A, Steinmetz MO. Control of microtubule organization and dynamics: two ends in the limelight. Nat Rev Mol Cell Biol 2015; 16:711-26; PMID:26562752; http://dx.doi.org/ 10.1038/nrm4084 [DOI] [PubMed] [Google Scholar]
- [32].Scholey JM. Kinesin-2: a family of heterotrimeric and homodimeric motors with diverse intracellular transport functions. Annu Rev Cell Dev Biol 2013; 29:443-69; PMID:23750925; http://dx.doi.org/ 10.1146/annurev-cellbio-101512-122335 [DOI] [PubMed] [Google Scholar]
- [33].Franker MA, Hoogenraad CC. Microtubule-based transport - basic mechanisms, traffic rules and role in neurological pathogenesis. J Cell Sci 2013; 126:2319-29; PMID:23729742; http://dx.doi.org/ 10.1242/jcs.115030 [DOI] [PubMed] [Google Scholar]
- [34].Scholey JM. Kinesin-II, a membrane traffic motor in axons, axonemes, and spindles. J Cell Biol 1996; 133:1-4; PMID:8601599; http://dx.doi.org/ 10.1083/jcb.133.1.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Ehrenberg M, McGrath JL. Actin motility: staying on track takes a little more effort. Curr Biol 2004; 14:R931-2; PMID:15530387; http://dx.doi.org/ 10.1016/j.cub.2004.10.018 [DOI] [PubMed] [Google Scholar]
- [36].Lie PPY, Mruk DD, Lee WM, Cheng CY. Cytoskeletal dynamics and spermatogenesis. Philos Trans R Soc Lond B Biol Sci 2010; 365:1581-92; PMID:20403871; http://dx.doi.org/ 10.1098/rstb.2009.0261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Blanchoin L, Boujemaa-Paterski R, Sykes C, Plastino J. Actin dynamics, architecture, and mechanics in cell motility. Physiol Rev 2014; 94:235-63; PMID:24382887; http://dx.doi.org/ 10.1152/physrev.00018.2013 [DOI] [PubMed] [Google Scholar]
- [38].Suarez C, Kovar DR. Internetwork competition for monomers governs actin cytoskeleton organization. Nat Rev Mol Cell Biol 2016; 17:799-810; PMID:27625321; http://dx.doi.org/19856169 10.1038/nrm.2016.106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Vogl AW, Vaid KS, Guttman JA. The Sertoli cell cytoskeleton. Adv Exp Med Biol 2008; 636:186-211; PMID:19856169; http://dx.doi.org/ 10.1007/978-0-387-09597-4_11 [DOI] [PubMed] [Google Scholar]
- [40].Cheng CY, Mruk DD. Cell junction dynamics in the testis: Sertoli-germ cell interactions and male contraceptive development. Physiol Rev 2002; 82:825-74; PMID:12270945; http://dx.doi.org/ 10.1152/physrev.00009.2002 [DOI] [PubMed] [Google Scholar]
- [41].Mruk DD, Cheng CY. Sertoli-Sertoli and Sertoli-germ cell interactions and their significance in germ cell movement in the seminiferous epithelium during spermatogenesis. Endocr Rev 2004; 25:747-806; PMID:15466940; http://dx.doi.org/ 10.1210/er.2003-0022 [DOI] [PubMed] [Google Scholar]
- [42].Foth BJ, Goedecke MC, Soldati D. New insights into myosin eveolution and classification. Proc Natl Acad Sci U S A 2006; 103:3681-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Hasson T, Walsh J, Cable J, Mooseker MS, Brown SD, Steel KP. Effects of shaker-1 mutations on mykosin-VIIa protein and mRNA expression. Cell Motil Cytoskeleton 1997; 37:127-38; PMID:9186010; http://dx.doi.org/ 10.1002/(SICI)1097-0169(1997)37:2%3c127::AID-CM5%3e3.0.CO;2-5 [DOI] [PubMed] [Google Scholar]
- [44].Wolfrum U, Liu X, Schmitt A, Udovichenko IP, Williams DS. Myosin VIIa as a common component of cilia and microvilli. Cell Motil Cytoskeleton 1998; 40:261-71; PMID:9678669; http://dx.doi.org/ 10.1002/(SICI)1097-0169(1998)40:3%3c261::AID-CM5%3e3.0.CO;2-G [DOI] [PubMed] [Google Scholar]
- [45].Hyenne V, Harf JC, Latz M, Maro B, Wolfrum U, Simmler MC. Vezatin, a ubiquitous protein of adherens cell-cell junctions, is exclusively expressed in germ cells in mouse testis. Reproduction 2007; 133:563-74; PMID:17379651; http://dx.doi.org/ 10.1530/REP-06-0271 [DOI] [PubMed] [Google Scholar]
- [46].Velichkova M, Guttman J, Warren C, Eng L, Kline K, Vogl A, et al. . A human homologue of Drosophila kelch associates with myosin-VIIa in specialized adhesion junctions. Cell Motil Cytoskeleton 2002; 51:147-64; PMID:11921171; http://dx.doi.org/ 10.1002/cm.10025 [DOI] [PubMed] [Google Scholar]
- [47].Lee NPY, Cheng CY. Ectoplasmic specialization, a testis-specific cell-cell actin-based adherens junction type: is this a potential target for male contraceptive development. Human Reprod Update 2004; 10:349-69; ; http://dx.doi.org/ 10.1093/humupd/dmh026 [DOI] [PubMed] [Google Scholar]
- [48].Sweeney HL, Houdusse A. Structural and functional insights into the myosin motor mechanism. Annu Rev Biophys 2010; 39:539-57; PMID:20192767; http://dx.doi.org/ 10.1146/annurev.biophys.050708.133751 [DOI] [PubMed] [Google Scholar]
- [49].Ruff C, Furch M, Brenner B, Manstein DJ, Meyhofer E. Single-molecule tracking of myosins with genetically engineered amplifier domains. Nat Struct Biol 2001; 8:226-9; PMID:11224566; http://dx.doi.org/ 10.1038/84962 [DOI] [PubMed] [Google Scholar]
- [50].Sakamoto T, Wang F, Schmitz S, Xu Y, Xu Q, Molloy JE, et al. . Neck length and processivity of myosin V. J Biol Chem 2003; 278:29201-0207; PMID:12740393; http://dx.doi.org/ 10.1074/jbc.M303662200 [DOI] [PubMed] [Google Scholar]
- [51].Krendel M, Mooseker MS. Myosins: tails (and heads) of functional diversity. Physiology 2005; 20:239-51; PMID:16024512; http://dx.doi.org/ 10.1152/physiol.00014.2005 [DOI] [PubMed] [Google Scholar]
- [52].Wu X, Rao K, Zhang H, Wang F, Sellers J, Matesic L, et al. . Identification of an organelle receptor for myosin-Va. Nat Cell Biol 2002; 4:271-8; PMID:11887186; http://dx.doi.org/ 10.1038/ncb760 [DOI] [PubMed] [Google Scholar]
- [53].Chen YM, Lee NPY, Mruk DD, Lee WM, Cheng CY. Fer kinase/Fer T and adherens junction dynamics in the testis: an in vitro and in vivo study. Biol Reprod 2003; 69:656-72; PMID:12700184; http://dx.doi.org/ 10.1095/biolreprod.103.016881 [DOI] [PubMed] [Google Scholar]
- [54].Cheng CY. Toxicants target cell junctions in the testis - insights from the indazole-carboxylic acid model. Spermatogenesis 2014; 4:e981485; PMID:26413399; http://dx.doi.org/ 10.4161/21565562.2014.981485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Cheng CY, Wong EWP, Lie PPY, Li MWM, Su L, Siu ER, et al. . Environmental toxicants and male reproductive function. Spermatogenesis 2011; 1:2-13; PMID:21866273; http://dx.doi.org/ 10.4161/spmg.1.1.13971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Johnson KJ. Testicular histopathology associated with disruption of the Sertoli cell cytoskeleton. Spermatogenesis 2014; 4:e979106; PMID:26413393; http://dx.doi.org/ 10.4161/21565562.2014.979106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Boekelheide K, Fleming SL, Allio T, Embree-Ku ME, Hall SJ, Johnson KJ, et al. . 2,5-Hexanedione-induced testicular injury. Annu Rev Pharmacol Toxciol 2003; 43:125-47; http://dx.doi.org/ 10.1146/annurev.pharmtox.43.100901.135930 [DOI] [PubMed] [Google Scholar]
- [58].Yan HHN, Mruk DD, Wong EWP, Lee WM, Cheng CY. An autocrine axis in the testis that coordinates spermiation and blood-testis barrier restructuring during spermatogenesis. Proc Natl Acad Sci USA 2008; 105:8950-5; PMID:18579774; http://dx.doi.org/ 10.1073/pnas.0711264105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Gao Y, Mruk DD, Lui WY, Lee WM, Cheng CY. F5-peptide induces aspermatogenesis by disrupting organization of actin- and microtubule-based cytoskeletons in the testis. Oncotarget (in press) 2016; PMID:27611949; http://dx.doi.org/23863697 10.18632/oncotarget1887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Dietrich S, Weiβ S, Pleiser S, Kerkhoff E. Structural and functional insights into the Spir/formin actin nucleator complex. Biological chemistry 2013; 394:1649-60; PMID:23863697; http://dx.doi.org/ 10.1515/hsz-2013-0176 [DOI] [PubMed] [Google Scholar]
- [61].Kerkhoff E. Actin dynamics at intracellular membranes: the Spir/formin nucleator complex. Eur J Cell Biol 2011; 90:922-5; PMID:21129813; http://dx.doi.org/ 10.1016/j.ejcb.2010.10.011 [DOI] [PubMed] [Google Scholar]
- [62].Campellone KG, Welch MD. A nucleator arms race: cellular control of actin assembly. Nat Rev Mol Cell Biol 2010; 11:237-51; PMID:20237478; http://dx.doi.org/ 10.1038/nrm2867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Mao Y. Formin a link between kinetochores and microtubule ends. Trends Cell Biol 2011; 21:625-9; PMID:21920754; http://dx.doi.org/ 10.1016/j.tcb.2011.08.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Tang EI, Mok KW, Lee WM, Cheng CY. EB1 regulates tubulin and actin cytoskeletal networks at the Sertoli cell blood-testis barrier in male rats - an in vitro study. Endocrinology 2015; 156:680-93; PMID:25456071; http://dx.doi.org/ 10.1210/en.2014-1720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Aivatiadou E, Ripolone M, Brunetti F, Berruti G. cAMP-Epac2-mediated activation of Rap1 in developing male germ cells: RA-RhoGAP as a possible direct down-stream effector. Mol Reprod Dev 2009; 76:407-16; PMID:18937323; http://dx.doi.org/ 10.1002/mrd.20963 [DOI] [PubMed] [Google Scholar]
- [66].Okada K, Miyake H, Yamaguchi K, Chiba K, Maeta K, Bilasy SE, et al. . Critical function of RA-GEF-2/Rapgef6, a guanine nucleotide exchange factor for Rap1, in mouse spermatogenesis. Biochem Biophys Res Commun 2014; 445:89-94; PMID:24491570; http://dx.doi.org/ 10.1016/j.bbrc.2014.01.149 [DOI] [PubMed] [Google Scholar]
- [67].Aivatiadou E, Mattei E, Ceriani M, Tilia L, Berruti G. Impaired fertility and spermiogenetic disorders with loss of cell adhesion in male mice expressing an interfering Rap1 mutant. Mol Biol Cell 2007; 18:1530-42; PMID:17314400; http://dx.doi.org/ 10.1091/mbc.E06-10-0902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Berruti G, Paiardi C. The dynamic of the apical ectoplasmic specialization between spermatids and Sertoli cells: the case of the small GTPase Rap1. BioMed research international 2014; 2014:635979; PMID:24719879; http://dx.doi.org/ 10.1155/2014/635979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].McKenney RJ, Huynh W, Tanenbaum ME, Bhabha G, Vale RD. Activation of cytoplasmic dynein motility by dynactin-cargo adapter complexes. Science 2015; 345:337-41; PMID:25035494; http://dx.doi.org/ 10.1126/science.1254198 [DOI] [PMC free article] [PubMed] [Google Scholar]


