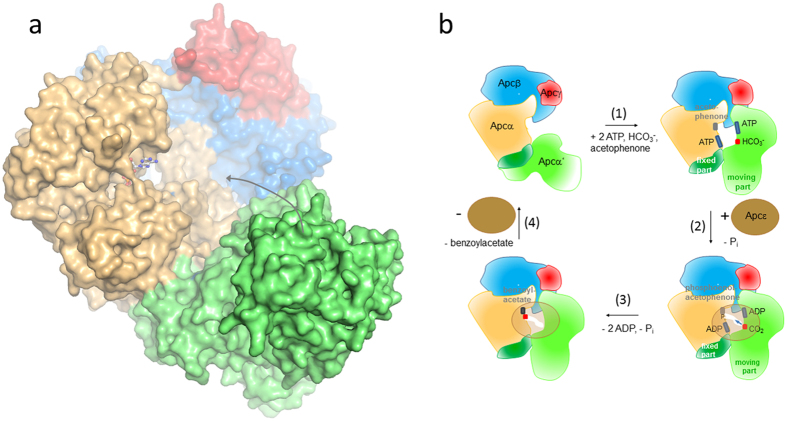
Figure 6. Scheme of the proposed enzymatic mechanism of Apc.
(a) Molecular surface representation of one Apcαα′βγ protomer in the structurally obtained open form. We postulate that upon substrate binding Apcα′ (green) moves towards the wide hollow formed by Apcα (beige), Apcβ (blue) and Apcγ (red). (b) Reaction cycle (1) Binding of ATP (blue) and acetophenone (grey) to Apcα (beige) as well as ATP (blue) and HCO3− (red) to Apcα′ induces a large conformational change of the apparently highly flexible part of Apcα′. (2) Binding of Apcε (brown) triggers the two kinase reactions. (3) Carboxyphosphate or the CO2 produced after hydrolysis diffuses via a formed channel (arrow) to the phosphoenol acetophenone, and the carboxylation reaction proceeds. (4) Dissociation of the Apc complex, product release and transition of the Apc core complex from closed to open.