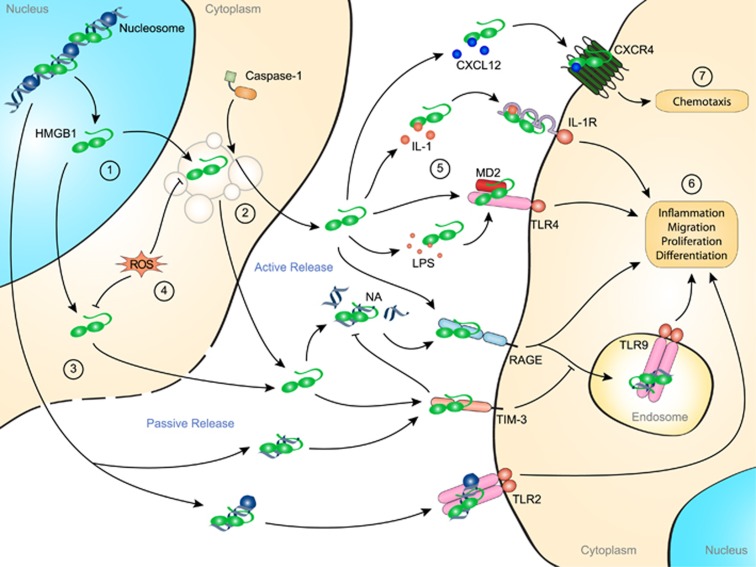
Figure 1.
Role of HMGB1 in inflammation. Under resting conditions, HMGB1 is localized in the nucleus, where it plays an important role in chromatin structure and gene expression. The translocation of HMGB1 to the cytoplasm is regulated by post-translational modifications such as acetylation, methylation and phosphorylation (1). Because of the lack of a secretion signal, HMGB1 is actively secreted through a caspase-1-dependent, noncanonical secretory vesicular pathway (2). HMGB1 can also be passively released from damaged cells either alone or in complex with RNA, DNA or nucleosomes (3). Interestingly, during apoptotic cell death, ROS production induces the terminal oxidation of HMGB1 that inhibits its proinflammatory function and switches HMGB1 function toward tolerogenicity (4). Once in the extracellular space, HMGB1 binds to several receptors in either free or complexed form (5). HMGB1 receptors, including RAGE and TLR4, bind free HMGB1 or HMGB1 in complex with DNA or LPS. Through its interaction with RAGE, the internalization of the HMGB1–DNA complex increases the activation of TLR9 localized in the endosome. However, HMGB1 complex formation with nucleic acids and potentially with other molecules can be inhibited by direct interaction with TIM-3. Other receptors, such as TLR2, IL-1R and CXCR4, recruit HMGB1 in complex with nucleosomes, IL-1β or CXCL12, respectively. Thus, sensing of HMGB1 mediates mechanisms of inflammation, cell migration, proliferation and differentiation (6). Furthermore, acting through the CXCL12/CXR4 axis, HMGB1 enhances chemotaxis (7). HMGB1, high-mobility group box 1 protein; IL, interleukin; LPS, lipopolysaccharide; RAGE, receptor for advanced glycation end-products; ROS, reactive oxygen species; TLR, Toll-like receptor.