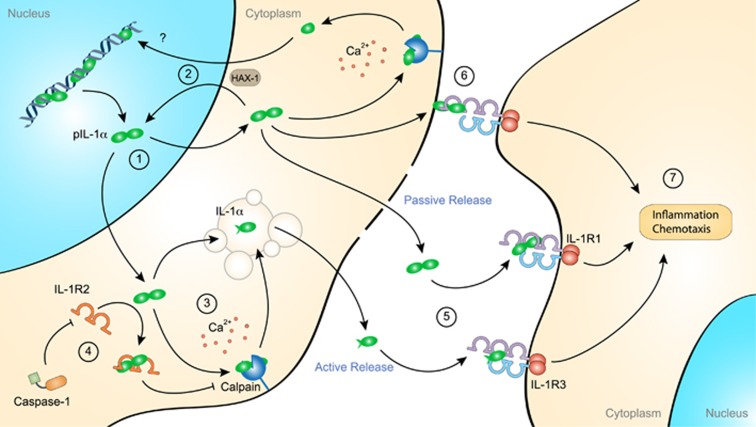
Figure 2.
Proinflammatory role of extracellular interleukin (IL)-1α. IL-1α precursor (pIL-1α) is constitutively expressed in most resting nonhematopoietic cells. In these cells, IL-1α is primarily localized in the nucleus, where it promotes gene expression by acting as a transcription factor (1). IL-1α nuclear transport depends on its interaction with HS1-associated protein X-1 (HAX-1) (2). In stimulated cells, pIL-1α is processed by the membrane-bound protease calpain, a calcium-dependent cysteine protease, before active release into the extracellular space through a noncanonical vesicular pathway (3). Interestingly, HAX-1 was also found to interact with the cleaved IL-1α N-terminal domain, suggesting its independent nuclear function (2). pIL-1α processing is inhibited by the cytosolic expression of IL-1 receptor-2 (IL-1R2) that binds to pIL-1α and thereby prevents its interaction with calpain and its subsequent secretion (4). However, active caspase-1 cleaves IL-1R2 and thereby enables pIL-1α processing. pIL-1α can also be passively released from damaged cells and, similar to mature IL-1α, interacts with IL-1 receptor-1 (IL-1R1) (5). In addition to being released in the extracellular space, IL-1α can be displayed at the plasma membrane, where it activates IL-1R1-expressing juxtaposing cells (6). Once sensed by IL-1R1, soluble or membrane-bound IL-1α induces the recruitment of the accessory receptor IL-1R3 and triggers proinflammatory signaling pathways (7).