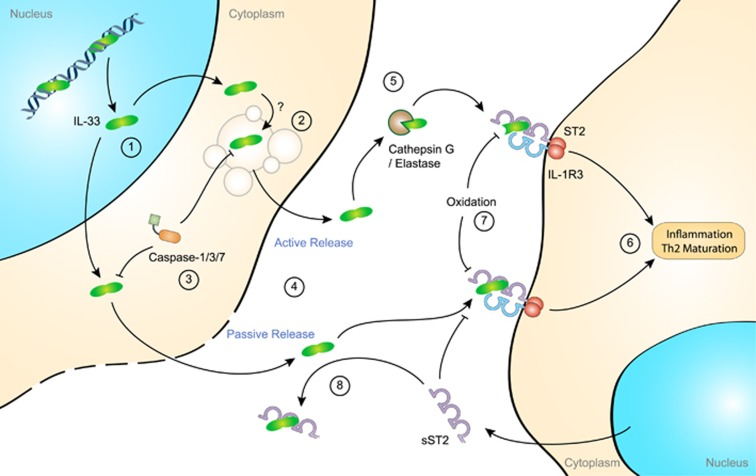
Figure 3.
Extracellular role of interleukin (IL)-33. IL-33 is present in the nucleus of most stromal cells of tissues in contact with the environment. In the nucleus, IL-33 represses gene expression by facilitating chromatin compaction. Little is known concerning IL-33 translocation to the cytoplasm (1). IL-33 lacks a secretion signal and, therefore, active release may require packaging in noncanonical secretory vesicles (2). Processing by caspases inhibits the proinflammatory function of IL-33 (3). It is therefore likely that IL-33 is released in its full-length form either actively or passively following cell damage (4). However, in the extracellular space, cleavage of IL-33 by neutrophil cathepsin G or elastase promotes its proinflammatory activity (5). Both full-length and cleaved IL-33 interact with the ST2 receptor and further recruit the accessory receptor, IL-1 receptor-3 (IL-1R3), to trigger proinflammatory signals or T helper 2 (Th2)-type cell maturation and response (6). Interestingly, IL-33 extracellular function is regulated in time and space by rapidly occurring oxidation that inhibits its binding to ST2 (7).