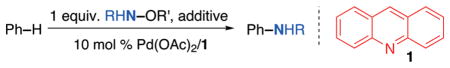
Table 1.
Reaction optimizationa
| ||||
|---|---|---|---|---|
| Entry | Additive | OR′ | R | Yield [%] |
| 1 | — | OAc | Ac | 11 |
| 2 | — | OAc | COCF3 | 21 |
| 3 | — | OAc | Ts | 13 |
| 4 | — | OTs | Ts | 10 |
| 5 | — | O2CCF3 | COCF3 | 22 |
| 6 | 1 equiv. AcOH | OAc | Ac | 29 |
| 7 | 5 mol% AgOAc | OAc | Ac | 27 |
| 8 | 1 equiv. AcOH | O2CCF3 | COCF3 | 17 |
| 9 | 5 mol% AgOAc | O2CCF3 | COCF3 | 22 |
| 10 | 1 equiv. AcOHb | OAc | Ac | 0 |
Conditions: benzene (0.50 mL, 5.6 mmol, 40 equiv.), Pd(OAc)2 (3.1 mg, 14 μmol, 10 mol%), 1 (2.5 mg, 14 μmol, 10 mol%), RNH–OR′ (140 μmol, 1.0 equiv.), 100 °C, 24 h.
No Pd catalyst.
