Figure 1.
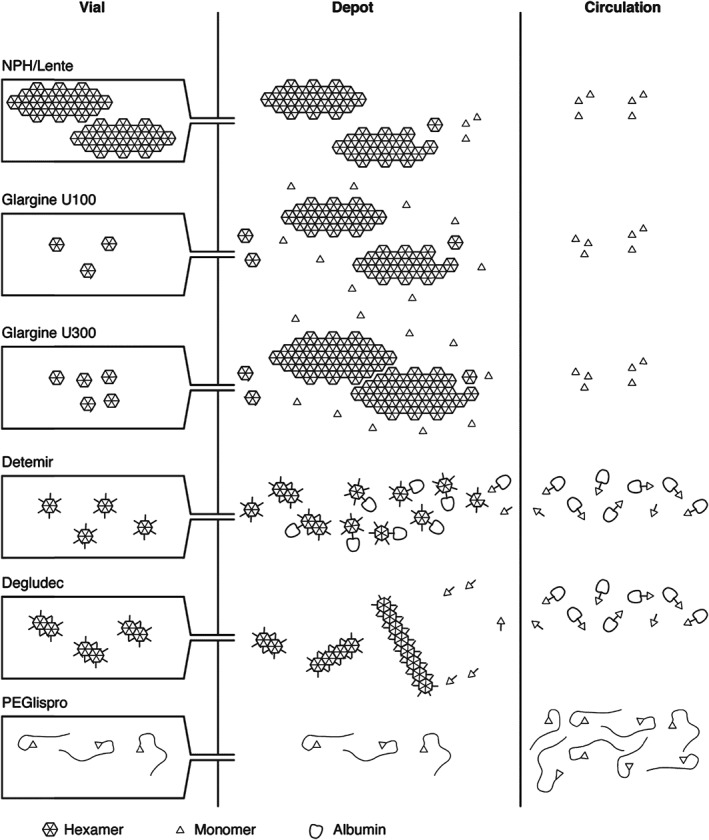
Summary of the different mechanisms of protraction. NPH insulin is injected as a pre‐formed protein–insulin conglomerate. On injection, the solvent from NPH insulin suspensions diffuses freely into the subcutaneous tissue but the crystals are retained in “heaps” at the injection depot. IGlar U100 is soluble in acidic formulation but, on subcutaneous injection and reaching physiological pH, it forms crystals. IGlar U300 also precipitates at physiological pH but these precipitates are much more compact compared with those of IGlar U100, so the surface area from which absorption can occur is reduced, thereby further slowing absorption. Acylation of IDet with a fatty acid side chain facilitates self‐association of IDet at the injection depot as dihexamers and reversible binding to albumin, both in the depot and in circulation, thereby slowing its absorption. IDeg also has a fatty acid side chain, which facilitates dihexamer formation in the vial and albumin binding in the circulation. However, protraction of absorption is primarily achieved via multihexamer chain formation in the depot. Subsequent dissociation of zinc causes the terminal hexamers to break down. The large hydrodynamic size of PEGlispro prolongs its action by slowing absorption and reducing clearance, effectively producing a circulating depot. The PEGlispro clinical trial programme was terminated in 2015.
