ABSTRACT
The tumor microenvironment is composed of many immune cell subpopulations and is an important factor in the malignant progression of neoplasms, particularly breast cancer (BC). However, the cytokine networks that coordinate various regulatory events within the BC interstitium remain largely uncharacterized. Moreover, the data obtained regarding the origin of cytokine secretions, the levels of secretion associated with tumor development, and the possible clinical relevance of cytokines remain controversial. Therefore, we profiled 27 cytokines in 78 breast tumor interstitial fluid (TIF) samples, 43 normal interstitial fluid (NIF) samples, and 25 matched serum samples obtained from BC patients with Luminex xMAP multiplex technology. Eleven cytokines exhibited significantly higher levels in the TIF samples compared with the NIF samples: interleukin (IL)-7, IL-10, fibroblast growth factor-2, IL-13, interferon (IFN)γ-inducible protein (IP-10), IL-1 receptor antagonist (IL-1RA), platelet-derived growth factor (PDGF)-β, IL-1β, chemokine ligand 5 (RANTES), vascular endothelial growth factor, and IL-12. An immunohistochemical analysis further demonstrated that IL-1RA, IP-10, IL-10, PDGF-β, RANTES, and VEGF are widely expressed by both cancer cells and tumor-infiltrating lymphocytes (TILs), whereas IP-10 and RANTES were preferentially abundant in triple-negative breast cancers (TNBCs) compared to Luminal A subtype cancers. The latter observation corresponds with the high level of TILs in the TNBC samples. IL-1β, IL-7, IL-10, and PDGFβ also exhibited a correlation between the TIF samples and matched sera. In a survival analysis, high levels of IL-5, a hallmark TH2 cytokine, in the TIF samples were associated with a worse prognosis. These findings have important implications for BC immunotherapy research.
KEYWORDS: Breast cancer, cytokine, growth factor, interleukin, interstitial fluid, tumor-infiltrating lymphocyte, TH2, array
Abbreviations
- ANOVA
analysis of variance
- BC
breast cancer
- DFS
disease-free survival
- FFPE
formalin-fixed, paraffin embedded
- ER
estrogen receptor
- FGF
fibroblast growth factor
- FISH
fluorescence in situ hybridization
- GM-CSF
granulocyte-macrophage colony-stimulating factor
- HER2
human epidermal growth factor receptor 2
- IFN
interferon
- IHC
immunohistochemistry
- IL
interleukin
- IP
inducible protein
- MCP-1
monocyte chemoattractant protein-1
- MIP
macrophage inflammatory protein
- NIF
normal interstitial fluid
- PDGF
platelet-derived growth factor
- PgR
progesterone receptor
- TAM
tumor-associated macrophage
- TIF
tumor interstitial fluid
- TILs
tumor-infiltrating lymphocytes
- TNBC
triple-negative breast cancer
- TNF
tumor-necrosis factor
- VEGF
vascular endothelial growth factor
Introduction
Breast cancer (BC) is currently the most commonly diagnosed form of female cancer with more than 1,300,000 cases diagnosed each year worldwide.1 It is also the leading cause of cancer-related deaths in women to date.1 It has been demonstrated that both the extensive genetic alterations that are observed in epithelial cancer cells2 and the composition of the stromal compartment can influence the progression of BC in a clinically relevant manner.3 These results highlight the complexity of this heterogeneous disease and also represent a major challenge in the development of targeted therapeutics. Accumulating evidence indicates that tumor growth and progression are dependent on the malignant potential of epithelial cancer cells and on the multidirectional interactions of factors produced by cell types that form a local tumor milieu. These include adipocytes, tumor-associated fibroblasts, endothelial cells, and immune cells. All of these cell types produce networks of cytokines and growth factors that are present in the local microenvironment.4-7 The importance of the tumor microenvironment in cancer growth and progression is widely accepted, yet the origin and significance of signaling cross-talk between cancer cells and the cells that constitute the supporting tumor interstitium, including immune cells, remains poorly understood. An important component of immune cells is the population of tumor-infiltrating lymphocytes (TILs). The presence of TILs is generally accepted as a prognostic factor for achieving a pathological complete response in BC patients following neoadjuvant chemotherapy (for a review see ref. [8]). Moreover, access of the peritumoural space and tumor islet by TILs has been shown to correlate with good prognosis in various cancers, including ovarian carcinoma,8 colon cancer,9, and BC.10,11
The complex composition of cell types in a tumor microenvironment enables a network of cytokines and growth factors to modulate the progression of malignant cells.12 Cytokine-mediated, multidirectional signaling events between cancer cells and leukocytes in the tumor-stroma milieu are generally implemented through the tumor interstitial fluid (TIF). Interstitial fluid forms at the interface between circulating bodily fluid and intracellular fluid, and provides an environment that facilitates the exchange of ions, proteins, cytokines, and growth factors between various cellular components within the interstitial space. Biomolecules that derive from cancer cells and stromal cells can also accumulate in TIF via secretion, exosome-mediated secretion, and membrane shedding. Thus, interstitial fluid represents a valuable resource for the discovery of novel biomarkers and therapeutic targets.12-14
Interstitial fluid may also provide insight into the regulatory mechanisms and functions of secretion-related processes during tumor development. The local tumor space accumulates secretome components at much higher concentrations compared with serum, and proximal lesion sampling and -omic profiling of tumor-associated fluid are two promising approaches for identifying novel candidate biomarkers.12 We previously developed a procedure for recovering TIF from fresh BC tissue specimens and performed a comprehensive, gel-based proteome characterization of BC interstitial fluids for a systematic search of potential biomarkers. As a result, a nine-protein signature profile with a higher abundance in TIF compared to normal counterparts was identified.12,15,16 Furthermore, in a preliminary study, a number of these cytokines were detected and measured in breast TIF using a cytokine-specific antibody array.16 A similar approach has been used by others to dissect the pathological role of interstitial molecules in cell migration, extracellular matrix reorganization, tumor microenvironment formation, morphogenesis, and immunity.12-14,17,18
Over the last decade, accumulating evidence has demonstrated a role for infiltrating leukocyte populations in BC progression.10,19 In contrast, very little is known about the in vivo origin of the cytokines associated with TILs, tumor subtypes, and clinical outcome. Here, we present the results of a comprehensive array-based analysis of 27 cytokines and growth factors in a large cohort of breast TIF, matched NIF, and serum samples. To our knowledge, this is the first large-scale study to profile various cytokines and growth factors secreted into the local interstitium of breast tumors in order to characterize a local cytokine response in a tumor microenvironment. This approach provides the basis for discriminating a systemic cytokine response that is induced by a primary cytokine reaction in a tumor niche and can be directly associated with malignancy. The main objectives of the present study were to: (i) identify and compare the abundance of cytokines and growth factors present in malignant versus normal interstitial fluids (NIFs); (ii) characterize the cytokine profiles of various tumor subtypes, (iii) identify a possible correlation between cytokines present in TIF with subpopulations of tumor-associated TILs, (iv) identify cytokines exhibiting a significant association with TIF and matched serum, and (v) identify a possible correlation between the cytokine profile of breast TIF and clinical outcome.
Results
Analysis of tumor-secreted cytokines and the tumor microenvironment: comparative cytokine profiling of TIF and NIF
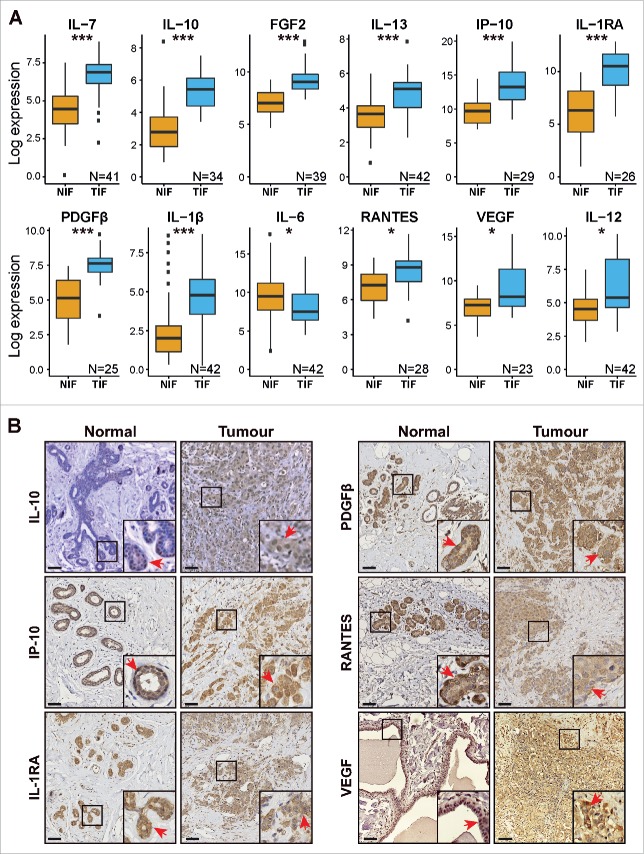
A quantitative comparison of the most prominent cytokines in breast TIF compared to NIF was performed. A total of 27 cytokines (Table S1) were measured across both sets of samples using a multiplex bead-based immune assay (Luminex). The amount of each sample that was loaded was normalized based on total protein concentration. The resulting cytokine concentrations detected in the proximal fluids were then log-transformed to achieve a similar data distribution across all of the samples. A paired analysis using matched samples identified 11 cytokines that were significantly elevated in the TIF samples compared with the NIF samples: interleukin (IL)-7, IL-10, fibroblast growth factor (FGF)-2, IL-13, interferon (IFN)γ-inducible protein (IP-10), IL-1 receptor antagonist (IL-1RA), platelet-derived growth factor (PDGF)-β, IL-1β, chemokine ligand 5 (RANTES), vascular endothelial growth factor (VEGF), and IL-12 (Fig. 1A). IL-6 was the only cytokine with a slight, yet significantly lower expression level in the TIF samples compared with the NIF samples (Fig. 1A).
Figure 1.
Differential abundance of cytokines in TIF and NIF samples. (A) Cytokines differentially presented in NIF and TIF samples. Pairs of samples with at least one missing value were excluded from this analysis. Paired t-test, adjusted p-values: *p < 0.05; ***p < 0.001. (B) IHC images showing expression of IL-10, IP-10, IL-1RA, PDGFβ, RANTES, and VEGF in representative pairs of tissue sections corresponding to the same NIF and TIF pair. Red arrows show positive staining in ductal epithelial cells within normal and malignant lesions. Scale bar = 100 µm.
To further characterize the origin and intra-tissue localization of differentially expressed cytokines, an immunohistochemical (IHC) analysis of selected tissue sections prepared from matched tumor and normal samples was performed. The tissue samples were selected based on the criterion of having high or low levels of the cytokines of interest detected in TIF samples compared to NIF samples, as well as the availability of corresponding tissue samples and specific antibodies. Thus, IHC staining was performed for IL-10, IP-10, IL-1RA, PDGFβ, RANTES, and VEGF (Fig. 1B). In Fig. S1, representative IHC results for several matched tumor/normal tissue samples are presented. Between 8 and 17 matched samples were stained for each of the 6 cytokines in order to confirm the similarity of the IHC patterns observed in the normal and tumor samples. A brief summary of the data is presented in a table at the bottom of Fig. S1. In the non-malignant breast tissue sections, very few infiltrating immune cells were observed (data not shown). Moreover, expression of IL-10, IP-10, IL-1RA, PDGFβ, RANTES, and VEGF was mainly restricted to the ductal epithelial cells (Fig. 1B), whereas their intracellular localization was primarily observed in the cytoplasm in both the luminal and basal cell layers. An exception was RANTES whose expression was substantially associated with the myoepithelial cells. In the stained tumor lesions, IP-10, IL-1RA, PDGFβ, RANTES, and VEGF exhibited moderate to strong staining intensities in a vast majority of the lesions analyzed (Fig. 1B, lower panel and Fig. S1). Furthermore, these cytokines often exhibited higher expression levels in the tumor cells than in the TILs (as shown for IP-10 and RANTES, Fig. 2). In contrast, expression of IL-10 was detected in ducts of the normal tissues, whereas the cancer cells exhibited a lower staining intensity compared to the other five cytokines that were assessed (Figs. 1B and S1).
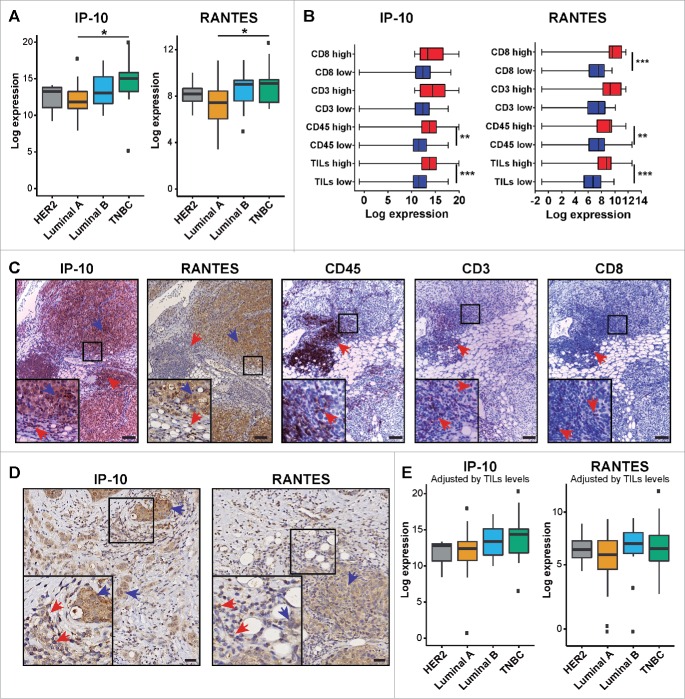
Figure 2.
Expression of IP-10 and RANTES among breast cancer subtypes. (A) Expression levels of IP-10 and RANTES according to tumor subtype; ANOVA test, *p < 0.05. (B) Expression of IP-10 and RANTES according to immune cell infiltration status; unpaired t-test **p < 0.01; ***p < 0.001. (C) Representative IHC images of serial sections showing the expression of IP-10 and RANTES in cancer cells (blue arrows) and in areas with lymphocyte infiltration (red arrows; CD45, CD3, and CD8+ markers) in a Luminal B tumor. Scale bar = 100 µm. (D) Representative IHC images showing cancer cells (blue arrows) and TILs (red arrows) expressing IP-10 and RANTES in a HER2 and Luminal B tumor section, respectively. Scale bar = 20 µm. (E) Expression levels of IP-10 and RANTES among tumor subtypes adjusted according to TILs infiltration using the ComBat function.
A complete list of all the samples analyzed in this study, including the histopathological, biochemical, and clinical parameters evaluated, is presented in Table S2.
Cytokines in the tumor interstitium were associated with breast tumor subtype and TILs
The role of TILs in BC subtypes has been found to be heterogeneous.20 Therefore, hematoxylin/eosin staining and IHC staining were performed to estimate the extent and type of lymphocyte infiltration present in the four major breast tumor subgroups identified among the lesions examined. First, the total number of TILs present in the tissue sections was scored with hematoxylin/eosin staining. Next, TIL subpopulations were characterized by performing IHC staining with antibodies specific for particular classes of lymphocytes: T-lymphocytes (anti-CD3 antibodies), T-helper lymphocytes (anti-CD4+ antibodies), cytotoxic T-lymphocytes (anti-CD8+ antibodies), and tumor-associated macrophages (TAMs) (anti-CD68 antibodies). The data listed in Table 1 show that Luminal A lesions had lower frequencies of TILs and CD3+ cells compared to the Luminal B and triple-negative breast cancer (TNBC) lesions. Similar results have been reported in other studies.10 In contrast, the levels of cytotoxic T lymphocytes (CD8+) detected were not statistically significant, whereas levels of TAMs (CD68+) significantly differed between the Luminal A and TNBC lesions (Table 1).
Table 1.
Association between breast cancer subtypes, total level of TILs, lymphocytes subpopulations, and macrophages
| Frequencies per tumor subtype, N (%)* |
||||||
|---|---|---|---|---|---|---|
| Immune cell subpopulation | HER2 | Lum A | Lum B | TNBC | Total | |
| TILs low | 2 (29) | 28 (72) | 7 (37) | 1 (8) | 38 (49) | |
| TILs high | 5 (71) | 11 (28) | 12 (63) | 12 (92) | 40 (51) | |
| CD3 low | 4 (80) | 36 (92) | 12 (66) | 7 (58) | 59 (80) | |
| CD3 high | 1 (20) | 3 (8) | 6 (34) | 5 (42) | 15 (20) | |
| CD4 low | 3 (50) | 33 (84) | 13 (72) | 5 (39) | 54 (71) | |
| CD4 high | 3 (50) | 6 (16) | 5 (28) | 8 (61) | 22 (29) | |
| CD8 low | 5 (83) | 35 (90) | 14 (77) | 9 (75) | 63 (84) | |
| CD8 high | 1 (17) | 4 (10) | 4 (23) | 3 (25) | 12 (16) | |
| CD68 low | 5 (83) | 31 (80) | 11 (61) | 5 (42) | 52 (69) | |
| CD68 high |
1 (17) |
8 (20) |
7 (39) |
7 (58) |
23 (31) |
|
|
p values** |
||||||
| Subtypes | TILs | CD3 | CD4 | CD8 | CD68 | |
| Lum A−LumB | 0.01 | 0.021 | ns | ns | ns | |
| Lum A−HER2 | 0.027 | ns | ns | ns | ns | |
| Lum A−TNBC | <0.001 | 0.012 | 0.002 | ns | 0.025 | |
| Lum B−HER2 | ns | ns | ns | ns | ns | |
| Lum B−TNBC | ns | ns | ns | ns | ns | |
| TNBC−HER2 | ns | ns | ns | ns | ns | |
Number of samples and percentage for each subtype are presented.
Fisher's exact test; ns = not significant.
We further analyzed the 27 cytokines across HER2, Luminal A, Luminal B, and TNBC subtypes. Significantly higher levels of IP-10 and RANTES were detected in the TNBC tissues than in the Luminal A tissues (Fig. 2A). As described above, the TNBC tissues analyzed in this study were characterized by a substantially higher rate of TILs compared to the Luminal A tissues (Table 1). The IP-10 and RANTES expression data were then categorized according to high versus low levels of TILs and CD3+, CD4+, and CD8+ TIL subsets across all four breast tumor subtypes. The tumors characterized by a high proportion of CD3+ TILs exhibited significant higher levels of IP-10 and RANTES than TIF samples with low CD3+ TILs (Fig. 2B). IHC staining of corresponding tissue sections further showed that expression of both IP-10 and RANTES were generally detected in tumor cells and TILs with relatively similar or slightly higher intensity (Fig. 2C and D), irrespective of tumor subtype. These findings imply that TILs may also contribute to the levels of soluble cytokines detected in breast TIF. When IP-10 and RANTES levels were corrected according to the TIL scoring that was performed with the ComBat function of the SVA package, greater similarity was observed among the patterns of IL-10 and RANTES expression for the various tumor subtypes (Fig. 2E). Thus, despite the data that show IP-10 and RANTES are expressed by tumor cells, TILs also appear to contribute to the total pool of secreted IL-10 and RANTES detected, particularly for the TNBC subtype.
Association of TIF cytokines with morphological and clinicopathological parameters
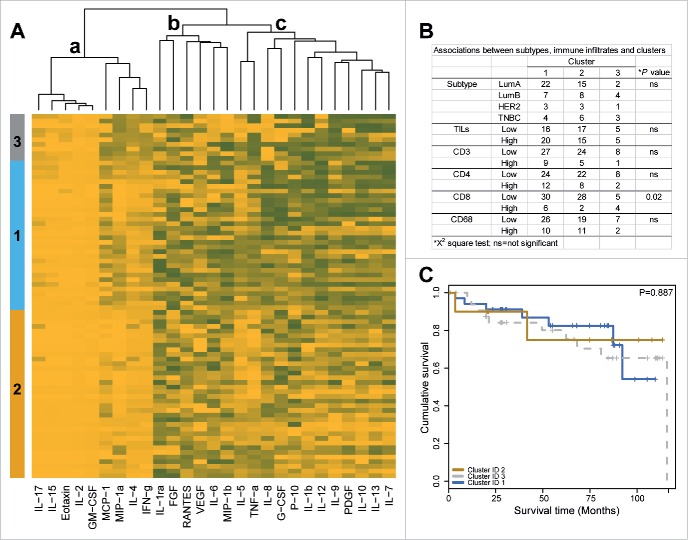
To identify potential associations between groups of cytokines with similar profiles and tumor subtype, immune cell infiltration and patient survival were subjected to unsupervised hierarchical clustering for all 27 cytokines of interest. TIF-associated cytokines were correlated by using k-means clustering. The corresponding heatmap is presented in Fig. 3A, and three major cytokine clusters are shown. Most of the cytokines that were present at low levels in the TIF samples (IL-17, IL15, eotaxin, IL-2, granulocyte-macrophage colony-stimulating factor (GM-CSF), monocyte chemoattractant protein-1 (MCP-1), macrophage inflammatory protein (MIP)-1A, IL-4, and IFNγ) were clustered (cluster a). Similarly, the cytokines that exhibited medium levels of expression were clustered (cluster b). A greater degree of correlation was observed among the cytokines that were highly abundant, namely IP-10, IL-1β, IL-12, IL-9, PDGFβ, IL-10, IL-13, and IL-7 (cluster c). All of the cytokines in cluster c were also identified as being highly abundant in the TIF samples compared with the NIF samples in the experiments described above (Fig. 1A). In particular, clusters 1 and 3 included TIF samples with high levels of cytokines (cytokine cluster c). In contrast, cluster 2 included samples with a lower abundance of cytokines. Cluster 3 was characterized by a higher infiltration of CD8+ cells, and no particular association with tumor subtype was observed (Fig. 3B). The clinicopathological parameters, such as tumor grade, patient age, tumor stage, and tumor size, did not significantly differ among the clusters. Furthermore, no association between disease-free survival (DFS) and the patient clusters were identified according to the log-rank test (Fig. 3C).
Figure 3.
Hierarchical clustering. (A) Heatmap of clustered cytokines (columns) and TIF samples (rows). Minimum and maximum normalized levels are shown in yellow and gray, respectively. k-means was used as the clustering method. (B) Association between TIF clusters, tumor subtypes, and immune cell subpopulations. (C) Kaplan–Meier plot illustrating DFS survival in patients with breast cancer according TIF clusters (N = 78), analyzed using a log-rank test.
Secretion of IL-5 in the tumor interstitium was associated with poor prognosis in the BC patients examined
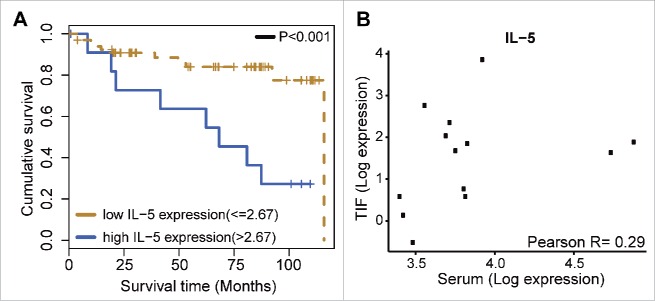
As emphasized above, it is well known that immune cells, particularly TILs, influence BC patient survival and therapy response.10 Considering that TILs also contribute to the secretion of cytokines into the tumor interstitium, we hypothesized that cytokines released by a tumor may influence immune signaling to affect tumor progression and disease course. To evaluate whether TIF cytokines are related to patient prognosis, a survival analysis was performed for the entire dataset of 27 cytokines. For this, the cytokines that were detected in the TIF samples were split into two groups according to their expression level (e.g., high versus low as described in Materials and Methods) and then were compared with DFS. A log-rank test analysis only identified a significant association for IL-5 (p < 0.001; Fig. 4A). The patients with high levels of IL-5 (n = 12) had a survival rate of 12%, with a median survival of 68.2 mo and a hazard ratio of 4.17. The patients with low levels of IL-5 (n = 66) had a 5-y survival rate of 92%, with a median survival of 115.8 mo. No association with survival has been found for each tumor subtype separately (data not shown). There was a modest trend for a positive correlation between higher levels of IL-5 in TIF and serum (Fig. 4B), thereby, implying that tumor-derived IL-5 could have a prognostic value in a serum analysis. However, no survival association was identified for serum levels of IL-5 (data not shown).
Figure 4.
IL-5 and breast cancer survival. (A) Kaplan–Meier DFS survival curves illustrating survival in patients with breast cancer according to IL-5 log-expression in TIF samples (N = 78). (B) Correlation analysis for IL-5 between TIF and serum levels (N = 13).
Correlation of cytokine levels in TIF and serum: the potential contribution of tumor-derived cytokines to the serum cytokine pool
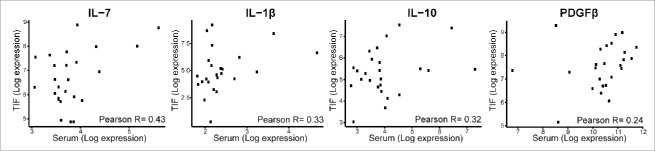
Previous studies have shown that certain cytokines are abundant in the serum of BC patients compared with the serum of healthy individuals.21 Studies in a mouse model of BC have also demonstrated that in the early stages of tumor progression, components of the tumor microenvironment gain access to the bloodstream.48 Both sets of results suggest that blood-based tests have the potential to detect a host’s response against a malignant tumor in its early stages. To investigate whether cytokines secreted into TIF contribute to cytokine levels in serum, Pearson’s correlation coefficient was applied to the cytokine data obtained from the TIF and NIF samples. A positive, yet modest, correlation was identified for IL-7, IL-1β, IL-10, and PDGFβ in the TIF samples compared with the NIF samples (Fig. 5). These results are consistent with the concept that serum levels of these cytokines are affected by the secretion of cytokines from the microenvironment of a tumor into the TIF and then into the blood.
Figure 5.
Correlation between cytokine levels in TIF and serum. Correlations were calculated using Pearson’s correlation coefficient (R).
Discussion
To the best of our knowledge, this study is the first to comprehensively profile a spectrum of various cytokines and growth factors in the local tumor interstitium of BC patients. A multiplex-array platform was used to comparatively assess a total of 27 cytokines and growth factors in interstitial fluid samples recovered from cancerous tissues (n = 78) and from corresponding normal tissues sampled from the vicinity of the cancer tissues (n = 43). This integrated approach allowed us to profile the cytokine landscape directly from the local tumor-environment space as a primary response to tumor metabolism, including inflammatory immune responses. These data also provide a basis for discriminating a local tumor response from systemic cytokine reactions that may be caused by stimuli not directly related to malignancy.
Eleven cytokines and growth factors were found to be consistently elevated in the breast TIF samples examined compared with the matched NIF samples. These included IL-7, IL-10, FGF2, IL-13, IP-10, IL-1RA, PDGFβ, IL-1β, RANTES, VEGF, and IL-12. Increased levels of these cytokine/growth factors in the tumor interstitium reflect the patients’ response to a growing tumor. When six of these cytokines were further examined in IHC analyses of available tissues, the contribution of these cytokines by immune cells proximal to the cancer cells appeared to be potentially greater than the contributions of the other stromal components to the total pool of cytokines. In the correlation analysis that was performed for all 27 cytokines across the four main breast subtypes characterized (i.e., HER2, Luminal A, Luminal B, and TNBC), levels of IP-10 and RANTES appeared to differentiate the TNBC subgroup from the Luminal A group. Furthermore, it should be noted that even though epithelial cancer cells in the TNBC lesions displayed high levels of both cytokines, the contribution of cytokines by the TILs to the total pool of secreted factors potentially accounts for the observed differences. However, the high levels of IL-1β, IL-1RA, IL-7, IL-10, IL-12, IL-13, FGF2, PDGFβ, and VEGF that were measured in the TIF samples suggest that these cytokines and growth factors were generated in a local tumor niche as a general response to tumor progression, independent of a specific association with immune subpopulations or tumor subtypes.
The available literature regarding a role for RANTES (CCL5) in BC is rather controversial. Tumor-derived RANTES has been associated with many clinical specimens of breast and cervical cancers and higher plasma levels of RANTES have been identified in patients with progressive and more advanced diseases than in patients in remission.22-24 Moreover, an analysis of core needle biopsies from 113 invasive BCs revealed that the mean concentration of RANTES was significantly higher in the group of patients with axillary lymph node metastasis compared with those without.25 In contrast, the results from two murine mammary tumor models did not show a correlation between tumor-derived RANTES expression and tumor growth rate or metastatic capacity.26,27 In the present study, elevated levels of RANTES in the tumor interstitium of the TNBC lesions were partly consistent with the results of a recent publication where TNBC cell invasiveness was found to be promoted by RANTES produced by breast peritumoural adipose tissue.28 Thus, additional large-scale studies are needed to determine the diagnostic and/or prognostic value of RANTES expression in BC patients.
Previous studies have shown that serum levels of IP-10 (CXCL10) are elevated in BC patients compared to controls,29 and also in patients with other malignancies.30,31 Here, we provide evidence that breast tumor tissues secrete more IP-10 than non-tumoral tissues in the same patient. Higher IP-10 secretion also correlated with T-cell infiltration, particularly in the TNBC subtype. Previously, positive IHC staining of IP-10 in BC sections correlated with a higher infiltration of T-cell lymphocytes (CD4+ and CD8+),32 thereby, suggesting a role for IP-10 in lymphocyte recruitment. Interestingly, experimental evidence has also demonstrated that IP-10 secretion by BC cells is a strong chemoattractant for regulatory T-cells (γδTreg). Correspondingly, in vivo neutralization of IP-10 has been found to inhibit the migration and trafficking of γδTreg into breast tumor sites.33 Importantly, Cxcl10 expression has been found to be of pivotal relevance for the efficacy of anthracycline treatments that induce the production of type I IFNs by malignant cells. For example, when the function of Cxcl10 was compromised via inactivation of mediators of its signaling pathway or via neutralization of its receptor, Cxcr3; anthracycline treatments did not achieve optimal therapeutic responses.34 The present data and those of others suggest that this may be due to the role of CXCL10 in lymphocyte recruitment. However, additional studies are needed to elucidate the details of this possible mechanism.
A subset of the cytokines analyzed in the present study has been shown to be related to the progression of BC and other cancer types. For example, PDGF signaling is recognized as being relevant for the cancer biology axis due to its experimentally documented effects on malignant cells and on other cells of the tumor microenvironment.35 In the present study, PDGFβ was found to be expressed in normal mammary gland tissues, particularly in the myoepithelial cell layer, and its expression was exacerbated in cancer cells and in other components of the tumor stroma, including immune cells. These results are consistent those of another study,36 and also highlight the role of expression levels of PDGFβ in relation to clinical outcome. For example, for tumors that express high levels of PDGFβ, both in vitro and in vivo inhibition of PDGFβ has been found to prevent pericyte loss and vascular permeability, thereby, leading to a decrease in metastasis formation.37
It was recently demonstrated that IL-1RA that is synthesized by Gr-1+ myeloid cells is able to prevent the onset of senescence in a PTEN-null prostate tumor model.38 In the same study, patients with high levels of IL-1RA did not respond to chemotherapy and experienced a shorter DFS period compared with patients with lower levels of IL-1RA.38 In the present study, IL-1RA was abundant in the TIF samples, with both cancer cells and TILs contributing to the high levels observed. However, we did not identify any association between IL-1RA levels and patient survival. The latter observation is most likely due to the relatively low number of samples available and the reduced number of events.
IL-7 is required for the normal development of T cells in mice and humans and is also needed for the maintenance of CD4+ and CD8+ T cells, thereby, promoting expansion of both naive and memory T cells.39 Early evidence showed that IL-7 was able to stimulate the proliferation of CD4+ TILs that were extracted from colorectal cancer biopsies.40 In normal breast tissues, low levels of IL-7 transcripts have been found, whereas IL-7 transcripts are generally absent in BC cell lines. In contrast, IL-7 receptor (IL7R) transcripts have been found in both BC cell lines and in normal breast tissue.41 Consistent with these previous observations, BC tissues were found to express higher levels of IL-7 than the normal breast tissues that were examined in the present study.
IL-10 is a molecule with immunosuppressive and immunostimulatory properties. In diffuse large B-cell lymphoma 42 and gastric cancer 43, elevated plasma levels of IL-10 have correlated with poor prognosis. A strong correlation between BC progression and IL-1β levels has also been observed.44 In a study by Kurtzman et al.45 elevated levels of IL-1β were observed in 90% of invasive BCs, with cellular localization of IL-1β observed in both cancer cells and stromal cells. In general, expression of IL-1β has been associated with more aggressive phenotypes in breast tumors.46,47
The use of inflammatory mediators as biomarkers is not straightforward since they are often present at higher levels in both cancers and non-neoplastic pathologies/conditions. However, certain inflammatory mediators may be generated as part of a general response to cancer. In mouse models of BC, cancer progression evokes a rapid physiological response from the tumor microenvironment, including immune response signaling. These changes induce a release of proteins into the plasma, including cytokines, angiogenic factors, and extracellular matrix components. Moreover, this release has been found to occur before the onset of a clinically detectable cancer.48 In this study, PDGFβ, IL-7, IL-1β, and IL-10 exhibited an association between their levels in TIF samples and their levels in matched sera. These results support the hypothesis that, for a subset of BC patients, an increase in serum levels of cytokines is due to the production of these cytokines within a tumor, thus, providing a readout of biological processes that are directly associated with cancer development/progression. Additional studies of large series of samples are needed to confirm these results and to determine their potential usefulness for achieving a reliable diagnosis of BC.
It has been well characterized that the activation of CD8+ cells is mediated by the Th1-response, and this process plays an important role in the treatment of BC either by conventional, or targeted, therapy in combination with radiotherapy.10,49 In contrast, a low density of T cells has been associated with poor prognosis for both colorectal cancer9,50 and BC.51 Here, high levels of IL-5 expression in TIF samples were identified as a factor in poor prognosis. IL-5 is a hallmark cytokine of the Th2 response that is associated with allergies and parasitic infections. IL-5 also has a prominent role in the promotion of B cell and eosinophil differentiation and proliferation.52 Correspondingly, cumulative evidence supports a critical role for IL-5 in cancer prognosis. In lung cancer models, depletion of IL-5 reduced metastasis, whereas the administration of recombinant IL-5 to IL-5 knockout mice significantly increased pulmonary metastasis.53 Similarly, exogenous administration of IL-5 to mice was found enhance malignant pleural effusions, a pathological consequence of cancer that is predominantly observed in lung and breast adenocarcinomas.54 In bladder cancer, IL-5 expression is associated with a muscle-invasive phenotype,55 whereas in vitro, IL-5 treatment increased the migration and invasion capacities of bladder cancer cells via the MMP-9/NF-κB/AP-1 pathway.56 A previous study also demonstrated that BCs with a higher metastatic capacity express significantly higher levels of IL-5 mRNA, and these results are consistent with the present results.57 In addition, it was recently shown that BC patients with high serum levels of IL-5 had a higher frequency of positive lymph nodes.58 The latter results are consistent with the present findings as well, and also suggest a role for IL-5 in BC metastasis. There was no association identified between serum levels of IL-5 and patient survival in the present study. However, it is possible that the relatively low number of samples available and the reduced number of events may have contributed to this result. In an independent BC cohort (MicMa),59,60 IL-5 levels were assessed using the same technology used in the present study and a non-significant tendency toward a bad prognosis was observed in patients with high serum levels of IL-5 expression (unpublished data, Jabeen et al., personal communication). Therefore, further studies are needed to confirm the role of IL-5 and patient prognosis.
In the present study, IL-4 was not identified as a prognosis factor, yet it is considered another hallmark modulator of the Th2 response.61 Moreover, similar to IL-5, a role for IL-4 in the promotion of invasive and metastatic behavior of BC cells has been proposed,62,63 thereby, supporting a role for Th2 signaling and its detrimental response. Enabling of a Th1 response appears to be related to a higher frequency of mutation rates in mismatch repair-deficient tumors, where it has been shown that mismatch-repair status predicts the clinical benefit of blocking immune checkpoints with pembrolizumab.64 This observation also strongly supports the hypothesis that a high number of mutation-associated neo-antigens are more likely to stimulate an immune response against a tumor. However, it remains unclear whether a low rate of mutations is sufficient to establish a Th2 response in tumors, or if this process depends on other mechanisms that have yet to be identified. Based on the evidence presented here that IL-5 is associated with a poor prognosis in BC cases, and the observations published by other authors that Th2 cells and other Th2-associated cytokines promote the invasion and metastasis,65 support for therapeutic strategies that inhibit or reverse the Th2 response in tumors to improve patient survival is provided.65
Conclusion
The exacerbated production and secretion of cytokines and growth factors by cancer cells and tumor-infiltrating immune cells is a consistent feature of BC tissues. Here, we provide evidence that tumor-infiltrating lymphocytes are contributors to the total pool of secreted cytokines, and in some cases, the extent of these secretions is BC subtype dependent. Furthermore, the leakage of tumor-produced cytokines into the bloodstream may account for the higher levels of certain cytokines in the serum of BC patients. Of particular interest is the finding that the intratumour levels of IL-5, a Th2- cytokine, were associated with poor prognosis in the group of BC patients that was examined. Consequently, further studies are needed to confirm and address the biological and clinical relevance of IL-5 in human BC.
Materials and Methods
Clinical samples: tumor tissues, matched non-malignant tissues, and serum
Fresh samples of tumor tissue and non-malignant tissue distant (about 5 cm) to the tumor margin were collected from patients defined as high risk according to the Danish Breast Cooperative Group (www.dbcg.dk, accessed 22.10.200916) that underwent a mastectomy between 2003 and 2012 as part of the Danish Center for Translational Breast Cancer Research program. All of the patients presented a unifocal tumor with an estimated size of more than 20 mm in diameter and none of the patients had a history of breast surgery or had received preoperative treatment. The age range of the selected cohort was 32–84 y (median age= 68.5 y). Patients were followed after surgery and cancer-specific survival was measured from the date of primary surgery until the date of death from BC. The date and cause of death were assigned in accordance with the Danish Cancer Registration System and the Danish Register of Cause of Death. Death records were complete up to October 08, 2014 and served as the censor date. Registered clinicopathological data for the patients were available from the Department of Pathology, Rigshospitalet, Copenhagen University Hospital, Denmark. This study was conducted in compliance with the Helsinki II Declaration and written informed consent was obtained from all participants. This project was approved by the Copenhagen and Frederiksberg regional division of the Danish National Committee on Biomedical Research Ethics (KF 01-069/03).
At the time of collection, each tumor biopsy and matched non-malignant tumor biopsy were divided into two pieces. One piece was stored at −80 °C and was subsequently prepared as a FFPE sample that was sectioned, mounted on glass slides, and stained for histological characterization, tumor subtyping, TIL scoring, and IHC studies. The second biopsy piece was placed in PBS at 4 °C within 30–45 min of surgical excision and then was subjected to interstitial fluid recovery (see below).
Matched sera were obtained from women that were enrolled in the Danish Center for Translational Breast Cancer Research program and underwent surgery between 2001 and 2006. Blood samples were collected preoperatively following a standardized protocol.66 The samples had only undergone one freeze/thaw cycle before they were analyzed.
Histological assessment of tissue biopsies: IHC and breast tumor subtyping
IHC analysis was performed as described previously to conduct histological characterizations of the tissue samples collected.16 First, small FFPE blocks were prepared from 2 to 3 various parts of the tissue piece and the sections were stained with a CK19 (KRT19) antibody. Tissue morphology and estimates of tumor cell content were made.15 A visual assessment of tumor-stroma percentages were evaluated as previously described.67 All of the slides were blindly reviewed (IIG, PSG).
Subtype scoring of the tumor tissues as Luminal A, Luminal B, HER2, or TNBC was performed based on the estrogen receptor (ER), progesterone receptor (PgR), HER2, and Ki67 status of each tissue in accordance with the St. Gallen International Breast Cancer Guidelines.68 For tumor stratification, the ER- and PgR-positive cases were considered negative when the percentage of nuclear immunoreactivity within the invasive cancer cells was <1%. The cases with ≥1% of the invasive cancer cells positively stained were classified as positive. Cases were considered HER2-positive if their membrane positivity was 3+ and/or the fluorescence in situ hybridization (FISH) ratio of HER2 to CEP17 was ≥2.0. For a HER2 IHC score of 2+, this was also evaluated by FISH and a value <2.0 was considered negative and a value ≥2.0 was considered positive. Mean Ki67 expression was used for subtype estimation, and the cutoff for Ki67 positivity was assigned in accordance with currently accepted criteria.69 Ki67 index values were measured using the open access web application, ImmunoRatio, to perform automated image analysis.70 The list of patients analyzed in this study, including sample type collected and tumor subtype identified, are presented in Table S2. In Table S4, the antibodies used in this study are listed. For tumor subtyping, antibodies recognizing ER, PgR, HER2, and Ki67 were used. For TIL subpopulation scoring, antibodies recognizing CD3, CD4+, CD8+, CD45, and CD68 were used. For cytokine detection, antibodies recognizing RANTES, PDGFβ, IP-10, IL-1RA, IL10, and VEGF were used. Standardization of the dilution, incubation, and development times appropriate for each antibody allowed an accurate comparison of expression levels in all cases. In all of the antibody staining studies conducted, positive and negative control slides were analyzed in parallel, with the latter incubated with PBS instead of primary antibodies.
Estimation of TILs and their subpopulations
The proportion of TILs in tissue sections was evaluated in accordance with the recommendations of the International TILs Working Group 2014.71 An assessment of overall inflammatory reactions and the number of lymphoid cells present within biopsies were determined for hematoxylin- and eosin-stained sections according to a previously described protocol 72 that included three categories for scoring of the stainings: (1+): absence of a lymphocyte infiltrate, (2+): partial infiltration by lymphocytes, and (3+): lymphocyte-predominant BC depending on the observed distribution of lymphocyte localization (see Fig. S2 and Table S2). IHC analyses were also performed to examine the most prominent components of the immune microenvironment in the breast tumors examined. The distribution of TILs was evaluated with IHC according to the detection of CD3+ cells, CD4+ cells, and CD8+ cells to identify T cells, helper T cells, and cytotoxic T cells, respectively. Scoring of these stainings was performed as previously reported 73-76, with the same cut-off criteria used for the positively stained cells as described above: 1+ (>10%), 2+ (10–50%), 3+ (>50%). These scores were independently and blindly assigned (IIG, PSG) and any discrepancies were resolved by consensus. The macrophage marker, CD68, was also evaluated with the same criteria. For each immune cell population that was analyzed, the expression results were dichotomized as low (<10%) and high (>10%).
Recovery of TIF
TIF and NIF samples were extracted from small surgically resected breast tumor pieces and from normal breast epithelial tissues that were collected proximal to the tumor cells, respectively, as previously described.77 Briefly, for each sample, approximately 0.1–0.3 g of clean tissue was cut into small pieces (~1 mm3 each), washed twice in cold PBS to remove blood and cell debris, and then incubated in PBS for 1 h at 37 °C in a humidified CO2 incubator. The samples then were centrifuged consecutively at 1,000 rpm and 5,000 rpm for 2 min and 20 min, respectively, each at 4 °C. After the supernatants were carefully aspirated, total protein concentration for each sample was determined with the Bradford assay.78
Luminex xMAP assay
A total of 27 cytokines, including ILs, chemokines, growth factors, IFN, and tumor necrosis factor (TNF), were analyzed in a 27-plex commercially available cytokine panel from Bio-Rad (Lot #: 5029511) (Table S1). Interstitial fluids obtained from 78 breast tumor tissues and 43 normal breast tissues, as well as 25 serum samples (see above), were analyzed. Total protein concentrations were determined for each sample in a series of control standard dilutions as instructed by the manufacturer. The same amount of each sample was than analyzed with the Luminex xMAP 200 platform. The results obtained were then collected and processed with Bio-Plex Manager 6.0 (Bio-Rad).
Data normalization and statistics
Statistical analysis was performed using the R statistical programming environment. For data normalization, the observed concentrations were log transformed using a pseudocount of 0.5. Next, the significant abundance of each cytokine in tumor samples versus normal samples was calculated using a paired t-test. p-values were adjusted for multiple hypothesis testing using Bonferroni correction. Associations between immune subpopulations (e.g., TILs and CD markers) and tumor subtypes were assessed using Fisher’s exact test and a χ2 test. Immune subpopulations with scores ≥2 and <2 were labeled as high and low, respectively. Correlation of cytokine levels between TIF and serum samples was computed using Pearson’s correlation coefficient. To address TILs as a source of variation for selected cytokines, TIF correction according to TIL status was performed using the ComBat function of the SVA package.79 The levels of cytokines were analyzed using ANOVA to test the difference of the mean between the tumor subtypes. Clustering of TIF samples according to cytokine levels was performed using k-means clustering with k = 3.
Survival analysis
To divide the samples assessed into groups according to high versus low cytokine secretion, the R-package MaxStat was used.80 A 10-fold cross-validation was then performed by dividing the data set into 10 parts and the cutoff value from 9 of the parts was used to assign a group label to the tumors of the 10th part. Survival analysis in R was also performed.81 Statistical significance of the curves obtained was determined by using the log-rank test. DFS was measured from the time of surgery until the date of first recurrence or the date of death from BC. The patients that survived or died due to other causes were censored.
Supplementary Material
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Acknowledgments
The authors wish to thank Maria Grønvig Nielsen for expert technical assistance, Professor Niels Kroman (Department of Breast Surgery, Copenhagen University Hospital, Denmark) and Professor Jiri Bartek (DCRC, Karolinska Institutet) for their invaluable support, and Niels Christian Christensen for help in retrieving clinical data from the Registry.
Funding
This work was supported by the Danish Cancer Society with grants received from the “A Race Against Breast Cancer” foundation, the John and Birthe Meyer Foundation, the Centre of Excellence: CARD (DNRF125), and by the Eurocan Platform grant. JAE was supported by FONDECYT, Fondo Nacional de Investigación y Tecnología (3140308) and Swedish Research Council. SJ was supported by a PhD fellowship of the South Eastern Norway Health Authority, no. 272904. Cytokine profiling was performed with a grant from Strategiske Ahus midler, no. 266972.
References
- 1.Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin 2011; 61:69-90; PMID:21296855; http://dx.doi.org/ 10.3322/caac.20107 [DOI] [PubMed] [Google Scholar]
- 2.TCGA-Network . Comprehensive molecular portraits of human breast tumours. Nature 2012; 490:61-70; PMID:23000897; http://dx.doi.org/ 10.1038/nature11412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Beck AH, Sangoi AR, Leung S, Marinelli RJ, Nielsen TO, van de Vijver MJ, West RB, van de Rijn M, Koller D. Systematic analysis of breast cancer morphology uncovers stromal features associated with survival. Sci Transl Med 2011; 3:108ra13; PMID:22072638; http://dx.doi.org/ 10.1126/scitranslmed.3002564 [DOI] [PubMed] [Google Scholar]
- 4.Whiteside TL. The tumor microenvironment and its role in promoting tumor growth. Oncogene 2008; 27:5904-12; PMID:18836471; http://dx.doi.org/ 10.1038/onc.2008.271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Polyak K, Haviv I, Campbell IG. Co-evolution of tumor cells and their microenvironment. Trends Genet 2009; 25:30-8; PMID:19054589; http://dx.doi.org/ 10.1016/j.tig.2008.10.012 [DOI] [PubMed] [Google Scholar]
- 6.Korkaya H, Liu S, Wicha MS. Breast cancer stem cells, cytokine networks, and the tumor microenvironment. J Clin Invest 2011; 121:3804-9; PMID:21965337; http://dx.doi.org/ 10.1172/JCI57099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Horimoto Y, Polanska UM, Takahashi Y, Orimo A. Emerging roles of the tumor-associated stroma in promoting tumor metastasis. Cell Adhes Migr 2012; 6:193-202; PMID:22568980; http://dx.doi.org/ 10.4161/cam.20631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Santoiemma PP, Powell DJ Jr. Tumor infiltrating lymphocytes in ovarian cancer. Cancer Biol Ther 2015; 16:807-20; PMID:25894333; http://dx.doi.org/ 10.1080/15384047.2015.1040960 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Galon J, Costes A, Sanchez-Cabo F, Kirilovsky A, Mlecnik B, Lagorce-Pages C, Tosolini M, Camus M, Berger A, Wind P et al.. Type, density, and location of immune cells within human colorectal tumors predict clinical outcome. Science 2006; 313:1960-4; PMID:17008531; http://dx.doi.org/ 10.1126/science.1129139 [DOI] [PubMed] [Google Scholar]
- 10.Kroemer G, Senovilla L, Galluzzi L, Andre F, Zitvogel L. Natural and therapy-induced immunosurveillance in breast cancer. Nat Med 2015; 21:1128-38; PMID:26444637; http://dx.doi.org/ 10.1038/nm.3944 [DOI] [PubMed] [Google Scholar]
- 11.Savas P, Salgado R, Denkert C, Sotiriou C, Darcy PK, Smyth MJ, Loi S. Clinical relevance of host immunity in breast cancer: from TILs to the clinic. Nat Rev Clin Oncol 2015; 13:228-241; PMID:26667975 http://dx.doi.org/ 10.1038/nrclinonc.2015.215 [DOI] [PubMed] [Google Scholar]
- 12.Gromov P, Gromova I, Olsen CJ, Timmermans-Wielenga V, Talman ML, Serizawa RR, Moreira JM. Tumor interstitial fluid – a treasure trove of cancer biomarkers. Biochim Biophys Acta 2013; 1834:2259-70; PMID:23416532; http://dx.doi.org/ 10.1016/j.bbapap.2013.01.013 [DOI] [PubMed] [Google Scholar]
- 13.Wagner M, Wiig H. Tumor interstitial fluid formation, characterization, and clinical implications. Front Oncol 2015; 5:115; PMID:26075182; http://dx.doi.org/ 10.3389/fonc.2015.00115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Haslene-Hox H, Tenstad O, Wiig H. Interstitial fluid-a reflection of the tumor cell microenvironment and secretome. Biochim Biophys Acta 2013; 1834:2336-46; PMID:23376185; http://dx.doi.org/ 10.1016/j.bbapap.2013.01.028 [DOI] [PubMed] [Google Scholar]
- 15.Gromov P, Gromova I, Bunkenborg J, Cabezon T, Moreira JM, Timmermans-Wielenga V, Roepstorff P, Rank F, Celis JE. Up-regulated proteins in the fluid bathing the tumour cell microenvironment as potential serological markers for early detection of cancer of the breast. Mol Oncol 2010; 4:65-89; PMID:20005186; http://dx.doi.org/ 10.1016/j.molonc.2009.11.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Celis JE, Gromov P, Cabezon T, Moreira JM, Ambartsumian N, Sandelin K, Rank F, Gromova I. Proteomic characterization of the interstitial fluid perfusing the breast tumor microenvironment: a novel resource for biomarker and therapeutic target discovery. Mol Cell Proteomics 2004; 3:327-44; PMID:14754989; http://dx.doi.org/ 10.1074/mcp.M400009-MCP200 [DOI] [PubMed] [Google Scholar]
- 17.Wiig H, Swartz MA. Interstitial fluid and lymph formation and transport: physiological regulation and roles in inflammation and cancer. Physiol Rev 2012; 92:1005-60; PMID:22811424; http://dx.doi.org/ 10.1152/physrev.00037.2011 [DOI] [PubMed] [Google Scholar]
- 18.Shieh AC, Swartz MA. Regulation of tumor invasion by interstitial fluid flow. Phys Biol 2011; 8:015012; PMID:21301060; http://dx.doi.org/ 10.1088/1478-3975/8/1/015012 [DOI] [PubMed] [Google Scholar]
- 19.Quigley DA, Kristensen V. Predicting prognosis and therapeutic response from interactions between lymphocytes and tumor cells. Mol Oncol 2015; 9:2054-62; PMID:26607741; http://dx.doi.org/ 10.1016/j.molonc.2015.10.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Loi S, Michiels S, Salgado R, Sirtaine N, Jose V, Fumagalli D, Kellokumpu-Lehtinen PL, Bono P, Kataja V, Desmedt C et al.. Tumor infiltrating lymphocytes are prognostic in triple negative breast cancer and predictive for trastuzumab benefit in early breast cancer: results from the FinHER trial. Ann Oncol 2014; 25:1544-50; PMID:24608200; http://dx.doi.org/ 10.1093/annonc/mdu112 [DOI] [PubMed] [Google Scholar]
- 21.Dehqanzada ZA, Storrer CE, Hueman MT, Foley RJ, Harris KA, Jama YH, Shriver CD, Ponniah S, Peoples GE. Assessing serum cytokine profiles in breast cancer patients receiving a HER2/neu vaccine using Luminex technology. Oncol Rep 2007; 17:687-94; PMID:17273752; http://dx.doi.org/ 10.3892/or.17.3.687 [DOI] [PubMed] [Google Scholar]
- 22.Azenshtein E, Luboshits G, Shina S, Neumark E, Shahbazian D, Weil M, Wigler N, Keydar I, Ben-Baruch A. The CC chemokine RANTES in breast carcinoma progression: regulation of expression and potential mechanisms of promalignant activity. Cancer Res 2002; 62:1093-102; PMID:11861388 [PubMed] [Google Scholar]
- 23.Niwa Y, Akamatsu H, Niwa H, Sumi H, Ozaki Y, Abe A. Correlation of tissue and plasma RANTES levels with disease course in patients with breast or cervical cancer. Clin Cancer Res 2001; 7:285-9; PMID:11234881 [PubMed] [Google Scholar]
- 24.Eissa SA, Zaki SA, El-Maghraby SM, Kadry DY. Importance of serum IL-18 and RANTES as markers for breast carcinoma progression. J Egypt Natl Canc Inst 2005; 17:51-5; PMID:16353083 [PubMed] [Google Scholar]
- 25.Sauer G, Schneiderhan-Marra N, Kazmaier C, Hutzel K, Koretz K, Muche R, Kreienberg R, Joos T, Deissler H. Prediction of nodal involvement in breast cancer based on multiparametric protein analyses from preoperative core needle biopsies of the primary lesion. Clin Cancer Res 2008; 14:3345-53; PMID:18519762; http://dx.doi.org/ 10.1158/1078-0432.CCR-07-4802 [DOI] [PubMed] [Google Scholar]
- 26.Jayasinghe MM, Golden JM, Nair P, O'Donnell CM, Werner MT, Kurt RA. Tumor-derived CCL5 does not contribute to breast cancer progression. Breast Cancer Res Treat 2008; 111:511-21; PMID:17978871; http://dx.doi.org/ 10.1007/s10549-007-9802-6 [DOI] [PubMed] [Google Scholar]
- 27.Agarwal A, Verma S, Burra U, Murthy NS, Mohanty NK, Saxena S. Flow cytometric analysis of Th1 and Th2 cytokines in PBMCs as a parameter of immunological dysfunction in patients of superficial transitional cell carcinoma of bladder. Cancer Immunol Immunother 2006; 55:734-43; PMID:16283306; http://dx.doi.org/ 10.1007/s00262-005-0045-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.D'Esposito V, Liguoro D, Ambrosio MR, Collina F, Cantile M, Spinelli R, Raciti GA, Miele C, Valentino R, Campiglia P et al.. Adipose microenvironment promotes triple negative breast cancer cell invasiveness and dissemination by producing CCL5. Oncotarget 2016; 17:24495-509; PMID:27027351; http://dx.doi.org/ 10.18632/oncotarget.8336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jafarzadeh A, Fooladseresht H, Nemati M, Assadollahi Z, Sheikhi A, Ghaderi A. Higher circulating levels of chemokine CXCL10 in patients with breast cancer: evaluation of the influences of tumor stage and chemokine gene polymorphism. Cancer Biomark 2016; 16:545-54; PMID:27002757; http://dx.doi.org/ 10.3233/CBM-160596 [DOI] [PubMed] [Google Scholar]
- 30.Polimeno M, Napolitano M, Costantini S, Portella L, Esposito A, Capone F, Guerriero E, Trotta A, Zanotta S, Pucci L et al.. Regulatory T cells, interleukin (IL)-6, IL-8, vascular endothelial growth factor (VEGF), CXCL10, CXCL11, epidermal growth factor (EGF) and hepatocyte growth factor (HGF) as surrogate markers of host immunity in patients with renal cell carcinoma. BJU Int 2013; 112:686-96; PMID:23495770; http://dx.doi.org/ 10.1111/bju.12068 [DOI] [PubMed] [Google Scholar]
- 31.Koshiol J, Castro F, Kemp TJ, Gao YT, Roa JC, Wang B, Nogueira L, Araya JC, Shen MC, Rashid A et al.. Association of inflammatory and other immune markers with gallbladder cancer: Results from two independent case-control studies. Cytokine 2016; 83:217-25; PMID:27173614; http://dx.doi.org/ 10.1016/j.cyto.2016.05.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mulligan AM, Raitman I, Feeley L, Pinnaduwage D, Nguyen LT, O'Malley FP, Ohashi PS, Andrulis IL. Tumoral lymphocytic infiltration and expression of the chemokine CXCL10 in breast cancers from the Ontario Familial Breast Cancer Registry. Clin Cancer Res 2013; 19:336-46; PMID:23213058; http://dx.doi.org/ 10.1158/1078-0432.CCR-11-3314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ye J, Ma C, Wang F, Hsueh EC, Toth K, Huang Y, Mo W, Liu S, Han B, Varvares MA et al.. Specific recruitment of gammadelta regulatory T cells in human breast cancer. Cancer Res 2013; 73:6137-48; PMID:23959855; http://dx.doi.org/ 10.1158/0008-5472.CAN-13-0348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sistigu A, Yamazaki T, Vacchelli E, Chaba K, Enot DP, Adam J, Vitale I, Goubar A, Baracco EE, Remédios C et al.. Cancer cell-autonomous contribution of type I interferon signaling to the efficacy of chemotherapy. Nat Med 2014; 20:1301-9; PMID:25344738; http://dx.doi.org/ 10.1038/nm.3708 [DOI] [PubMed] [Google Scholar]
- 35.Paulsson J, Ehnman M, Ostman A. PDGF receptors in tumor biology: prognostic and predictive potential. Future Oncol 2014; 10:1695-708; PMID:25145436; http://dx.doi.org/ 10.2217/fon.14.83 [DOI] [PubMed] [Google Scholar]
- 36.Yokoyama Y, Mori S, Hamada Y, Hieda M, Kawaguchi N, Shaker M, Tao Y, Yoshidome K, Tsujimoto M, Matsuura N. Platelet-derived growth factor regulates breast cancer progression via beta-catenin expression. Pathobiology 2011; 78:253-60; PMID:21849806; http://dx.doi.org/ 10.1159/000328061 [DOI] [PubMed] [Google Scholar]
- 37.Hosaka K, Yang Y, Seki T, Nakamura M, Andersson P, Rouhi P, Yang X, Jensen L, Lim S, Feng N et al.. Tumour PDGF-BB expression levels determine dual effects of anti-PDGF drugs on vascular remodelling and metastasis. Nat Commun 2013; 4:2129; PMID:23831851; http://dx.doi.org/ 10.1038/ncomms3129 [DOI] [PubMed] [Google Scholar]
- 38.Di Mitri D, Toso A, Chen JJ, Sarti M, Pinton S, Jost TR, D'Antuono R, Montani E, Garcia-Escudero R, Guccini I et al.. Tumour-infiltrating Gr-1+ myeloid cells antagonize senescence in cancer. Nature 2014; 515:134-7; PMID:25156255; http://dx.doi.org/ 10.1038/nature13638 [DOI] [PubMed] [Google Scholar]
- 39.Sportes C, Hakim FT, Memon SA, Zhang H, Chua KS, Brown MR, Fleisher TA, Krumlauf MC, Babb RR, Chow CK et al.. Administration of rhIL-7 in humans increases in vivo TCR repertoire diversity by preferential expansion of naive T cell subsets. J Exp Med 2008; 205:1701-14; PMID:18573906; http://dx.doi.org/ 10.1084/jem.20071681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Maeurer MJ, Walter W, Martin D, Zitvogel L, Elder E, Storkus W, Lotze MT. Interleukin-7 (IL-7) in colorectal cancer: IL-7 is produced by tissues from colorectal cancer and promotes preferential expansion of tumour infiltrating lymphocytes. Scand J Immunol 1997; 45:182-92; PMID:9042431; http://dx.doi.org/ 10.1046/j.1365-3083.1997.d01-384.x [DOI] [PubMed] [Google Scholar]
- 41.Al-Rawi MA, Rmali K, Watkins G, Mansel RE, Jiang WG. Aberrant expression of interleukin-7 (IL-7) and its signalling complex in human breast cancer. Eur J Cancer 2004; 40:494-502; PMID:14962714; http://dx.doi.org/ 10.1016/j.ejca.2003.10.016 [DOI] [PubMed] [Google Scholar]
- 42.Lech-Maranda E, Bienvenu J, Michallet A-S, Houot R, Robak T, Coiffier B, Salles G. Elevated IL-10 plasma levels correlate with poor prognosis in diffuse large B-cell lymphoma. Eur Cytokine Netw 2006; 17:60-6; PMID:16613764 [PubMed] [Google Scholar]
- 43.Ock CY, Nam AR, Bang JH, Kim TY, Lee KH, Han SW, Im SA, Kim TY, Bang YJ, Oh DY. Signature of cytokines and angiogenic factors (CAFs) defines a clinically distinct subgroup of gastric cancer. Gastric Cancer 2015; 1-11; PMID:26681196; http://dx.doi.org/12851675 10.1007/s10120-015-0583-z [DOI] [PubMed] [Google Scholar]
- 44.Pantschenko AG, Pushkar I, Anderson KH, Wang Y, Miller LJ, Kurtzman SH, Barrows G, Kreutzer DL. The interleukin-1 family of cytokines and receptors in human breast cancer: implications for tumor progression. Int J Oncol 2003; 23:269-84; PMID:12851675; http://dx.doi.org/ 10.3892/ijo.23.2.269 [DOI] [PubMed] [Google Scholar]
- 45.Kurtzman SH, Anderson KH, Wang Y, Miller LJ, Renna M, Stankus M, Lindquist RR, Barrows G, Kreutzer DL. Cytokines in human breast cancer: IL-1alpha and IL-1beta expression. Oncol Rep 1999; 6:65-70; PMID:9864403; http://dx.doi.org/ 10.3892/or.6.1.65 [DOI] [PubMed] [Google Scholar]
- 46.Chavey C, Bibeau F, Gourgou-Bourgade S, Burlinchon S, Boissiere F, Laune D, Roques S, Lazennec G. Oestrogen receptor negative breast cancers exhibit high cytokine content. Breast Cancer Res 2007; 9:R15; PMID:17261184; http://dx.doi.org/ 10.1186/bcr1648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jin L, Yuan RQ, Fuchs A, Yao Y, Joseph A, Schwall R, Schnitt SJ, Guida A, Hastings HM, Andres J et al.. Expression of interleukin-1beta in human breast carcinoma. Cancer 1997; 80:421-34; PMID:9241076; http://dx.doi.org/ 10.1002/(SICI)1097-0142(19970801)80:3%3c421::AID-CNCR10%3e3.0.CO;2-Z [DOI] [PubMed] [Google Scholar]
- 48.Pitteri SJ, Kelly-Spratt KS, Gurley KE, Kennedy J, Buson TB, Chin A, Wang H, Zhang Q, Wong CH, Chodosh LA et al.. Tumor microenvironment-derived proteins dominate the plasma proteome response during breast cancer induction and progression. Cancer Res 2011; 71:5090-100; PMID:21653680; http://dx.doi.org/ 10.1158/0008-5472.CAN-11-0568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Callari M, Musella V, Di Buduo E, Sensi M, Miodini P, Dugo M, Orlandi R, Agresti R, Paolini B, Carcangiu ML et al.. Subtype-dependent prognostic relevance of an interferon-induced pathway metagene in node-negative breast cancer. Mol Oncol 2014; 8:1278-89; PMID:24853384; http://dx.doi.org/ 10.1016/j.molonc.2014.04.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pages F, Berger A, Camus M, Sanchez-Cabo F, Costes A, Molidor R, Mlecnik B, Kirilovsky A, Nilsson M, Damotte D et al.. Effector memory T cells, early metastasis, and survival in colorectal cancer. N Engl J Med 2005; 353:2654-66; PMID:16371631; http://dx.doi.org/ 10.1056/NEJMoa051424 [DOI] [PubMed] [Google Scholar]
- 51.Gu-Trantien C, Loi S, Garaud S, Equeter C, Libin M, de Wind A, Ravoet M, Le Buanec H, Sibille C, Manfouo-Foutsop G et al.. CD4(+) follicular helper T cell infiltration predicts breast cancer survival. J Clin Invest 2013; 123:2873-92; PMID:23778140; http://dx.doi.org/ 10.1172/JCI67428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Takatsu K. Interleukin-5 and IL-5 receptor in health and diseases. Proc Jpn Acad Ser B Phys Biol Sci 2011; 87:463-85; PMID:21986312; http://dx.doi.org/ 10.2183/pjab.87.463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zaynagetdinov R, Sherrill TP, Gleaves LA, McLoed AG, Saxon JA, Habermann AC, Connelly L, Dulek D, Peebles RS Jr, Fingleton B et al.. Interleukin-5 facilitates lung metastasis by modulating the immune microenvironment. Cancer Res 2015; 75:1624-34; PMID:25691457; http://dx.doi.org/ 10.1158/0008-5472.CAN-14-2379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Stathopoulos GT, Sherrill TP, Karabela SP, Goleniewska K, Kalomenidis I, Roussos C, Fingleton B, Yull FE, Peebles RS Jr, Blackwell TS. Host-derived interleukin-5 promotes adenocarcinoma-induced malignant pleural effusion. Am J Respir Crit Care Med 2010; 182:1273-81; PMID:20595227; http://dx.doi.org/ 10.1164/rccm.201001-0001OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lee SJ, Lee EJ, Kim SK, Jeong P, Cho YH, Yun SJ, Kim S, Kim GY, Choi YH, Cha EJ et al.. Identification of pro-inflammatory cytokines associated with muscle invasive bladder cancer; the roles of IL-5, IL-20, and IL-28A. PLoS One 2012; 7:e40267; PMID:22962576; http://dx.doi.org/ 10.1371/journal.pone.0040267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lee EJ, Lee SJ, Kim S, Cho SC, Choi YH, Kim WJ, Moon SK. Interleukin-5 enhances the migration and invasion of bladder cancer cells via ERK1/2-mediated MMP-9/NF-kappaB/AP-1 pathway: involvement of the p21WAF1 expression. Cell Signal 2013; 25:2025-38; PMID:23770289; http://dx.doi.org/ 10.1016/j.cellsig.2013.06.004 [DOI] [PubMed] [Google Scholar]
- 57.Eiro N, Gonzalez L, Gonzalez LO, Fernandez-Garcia B, Lamelas ML, Marin L, González-Reyes S, del Casar JM, Vizoso FJ. Relationship between the inflammatory molecular profile of breast carcinomas and distant metastasis development. PLoS One 2012; 7:e49047; PMID:23145063; http://dx.doi.org/ 10.1371/journal.pone.0049047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Konig A, Vilsmaier T, Rack B, Friese K, Janni W, Jeschke U, Andergassen U, Trapp E, Jückstock J, Jäger B et al.. Determination of interleukin-4, -5, -6, -8 and -13 in serum of patients with breast cancer before treatment and its correlation to circulating tumor cells. Anticancer Res 2016; 36:3123-30; PMID:27272837 [PubMed] [Google Scholar]
- 59.Naume B, Zhao X, Synnestvedt M, Borgen E, Russnes HG, Lingjaerde OC, Strømberg M, Wiedswang G, Kvalheim G, Kåresen R et al.. Presence of bone marrow micrometastasis is associated with different recurrence risk within molecular subtypes of breast cancer. Mol Oncol 2007; 1:160-71; PMID:19383292; http://dx.doi.org/ 10.1016/j.molonc.2007.03.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ronneberg JA, Fleischer T, Solvang HK, Nordgard SH, Edvardsen H, Potapenko I, Nebdal D, Daviaud C, Gut I, Bukholm I, et al.. Methylation profiling with a panel of cancer related genes: association with estrogen receptor, TP53 mutation status and expression subtypes in sporadic breast cancer. Mol Oncol 2011; 5:61-76; PMID:21212030; http://dx.doi.org/ 10.1016/j.molonc.2010.11.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wynn TA. Type 2 cytokines: mechanisms and therapeutic strategies. Nat Rev Immunol 2015; 15:271-82; PMID:25882242; http://dx.doi.org/ 10.1038/nri3831 [DOI] [PubMed] [Google Scholar]
- 62.DeNardo DG, Barreto JB, Andreu P, Vasquez L, Tawfik D, Kolhatkar N, Coussens LM. CD4(+) T cells regulate pulmonary metastasis of mammary carcinomas by enhancing protumor properties of macrophages. Cancer Cell 2009; 16:91-102; PMID:19647220; http://dx.doi.org/ 10.1016/j.ccr.2009.06.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zhang Q, Qin J, Zhong L, Gong L, Zhang B, Zhang Y, Gao WQ. CCL5-Mediated Th2 immune polarization promotes metastasis in luminal breast cancer. Cancer Res 2015; 75:4312-21; PMID:26249173; http://dx.doi.org/ 10.1158/0008-5472.CAN-14-3590 [DOI] [PubMed] [Google Scholar]
- 64.Le DT, Uram JN, Wang H, Bartlett BR, Kemberling H, Eyring AD, Skora AD, Luber BS, Azad NS, Laheru D et al.. PD-1 blockade in tumors with mismatch-repair deficiency. N Engl J Med 2015; 372:2509-20; PMID:26028255; http://dx.doi.org/ 10.1056/NEJMoa1500596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Palucka AK, Coussens LM. The basis of oncoimmunology. Cell 2016; 164:1233-47; PMID:26967289; http://dx.doi.org/ 10.1016/j.cell.2016.01.049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wurtz SO, Moller S, Mouridsen H, Hertel PB, Friis E, Brunner N. Plasma and serum levels of tissue inhibitor of metalloproteinases-1 are associated with prognosis in node-negative breast cancer: a prospective study. Mol Cell Proteomics 2008; 7:424-30; PMID:17998244; http://dx.doi.org/ 10.1074/mcp.M700305-MCP200 [DOI] [PubMed] [Google Scholar]
- 67.Mesker WE, Junggeburt JM, Szuhai K, de Heer P, Morreau H, Tanke HJ, Tollenaar RA. The carcinoma-stromal ratio of colon carcinoma is an independent factor for survival compared to lymph node status and tumor stage. Cell Oncol 2007; 29:387-98; PMID:17726261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Esposito A, Criscitiello C, Curigliano G. Highlights from the 14(th) St Gallen International Breast Cancer Conference 2015 in Vienna: Dealing with classification, prognostication, and prediction refinement to personalize the treatment of patients with early breast cancer. Ecancermedicalscience 2015; 9:518; PMID:25932042; http://dx.doi.org/ 10.3332/ecancer.2015.518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Goldhirsch A, Wood WC, Coates AS, Gelber RD, Thurlimann B, Senn HJ. Strategies for subtypes-dealing with the diversity of breast cancer: highlights of the St. Gallen International Expert Consensus on the Primary Therapy of Early Breast Cancer 2011. Ann Oncol 2011; 22:1736-47; PMID:21709140; http://dx.doi.org/ 10.1093/annonc/mdr304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Tuominen VJ, Ruotoistenmaki S, Viitanen A, Jumppanen M, Isola J. ImmunoRatio: a publicly available web application for quantitative image analysis of estrogen receptor (ER), progesterone receptor (PR), and Ki-67. Breast Cancer Res 2010; 12:R56; PMID:20663194; http://dx.doi.org/ 10.1186/bcr2615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Salgado R, Denkert C, Demaria S, Sirtaine N, Klauschen F, Pruneri G, Wienert S, Van den Eynden G, Baehner FL, Penault-Llorca F et al.. The evaluation of tumor-infiltrating lymphocytes (TILs) in breast cancer: recommendations by an International TILs Working Group 2014. Ann Oncol 2015; 26:259-71; PMID:25214542; http://dx.doi.org/19917869 10.1093/annonc/mdu450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Denkert C, Loibl S, Noske A, Roller M, Muller BM, Komor M, Budczies J, Darb-Esfahani S, Kronenwett R, Hanusch C et al.. Tumor-associated lymphocytes as an independent predictor of response to neoadjuvant chemotherapy in breast cancer. J Clin Oncol 2010; 28:105-13; PMID:19917869; http://dx.doi.org/ 10.1200/JCO.2009.23.7370 [DOI] [PubMed] [Google Scholar]
- 73.Klintrup K, Makinen JM, Kauppila S, Vare PO, Melkko J, Tuominen H, Tuppurainen K, Mäkelä J, Karttunen TJ, Mäkinen MJ. Inflammation and prognosis in colorectal cancer. Eur J Cancer 2005; 41:2645-54; PMID:16239109; http://dx.doi.org/ 10.1016/j.ejca.2005.07.017 [DOI] [PubMed] [Google Scholar]
- 74.Mohamed MM, El-Ghonaimy EA, Nouh MA, Schneider RJ, Sloane BF, El-Shinawi M. Cytokines secreted by macrophages isolated from tumor microenvironment of inflammatory breast cancer patients possess chemotactic properties. Int J Biochem Cell Biol 2014; 46:138-47; PMID:24291763; http://dx.doi.org/ 10.1016/j.biocel.2013.11.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Garcia-Martinez E, Gil GL, Benito AC, Gonzalez-Billalabeitia E, Conesa MA, Garcia Garcia T, García-Garre E, Vicente V, Ayala de la Peña F. Tumor-infiltrating immune cell profiles and their change after neoadjuvant chemotherapy predict response and prognosis of breast cancer. Breast Cancer Res 2014; 16:488; PMID:25432519; http://dx.doi.org/ 10.1186/s13058-014-0488-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Gujam FJ, Edwards J, Mohammed ZM, Going JJ, McMillan DC. The relationship between the tumour stroma percentage, clinicopathological characteristics and outcome in patients with operable ductal breast cancer. Br J Cancer 2014; 111:157-65; PMID:24874480; http://dx.doi.org/ 10.1038/bjc.2014.279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Celis JE, Cabezon T, Moreira JM, Gromov P, Gromova I, Timmermans-Wielenga V, Iwase T, Akiyama F, Honma N, Rank F. Molecular characterization of apocrine carcinoma of the breast: validation of an apocrine protein signature in a well-defined cohort. Mol Oncol 2009; 3:220-37; PMID:19393583; http://dx.doi.org/ 10.1016/j.molonc.2009.01.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 1976; 72:248-54; PMID:942051; http://dx.doi.org/ 10.1016/0003-2697(76)90527-3 [DOI] [PubMed] [Google Scholar]
- 79.Leek JT, Storey JD. Capturing heterogeneity in gene expression studies by surrogate variable analysis. PLoS Genet 2007; 3:1724-35; PMID:17907809; http://dx.doi.org/ 10.1371/journal.pgen.0030161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Hothorn T, Lausen B. On the exact distribution of maximally selected rank statistics. Comput Stat Data Anal 2003; 43:121-37; http://dx.doi.org/ 10.1016/S0167-9473(02)00225-6 [DOI] [Google Scholar]
- 81.Therneau T. A Package for Survival Analysis in S. 2015. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.