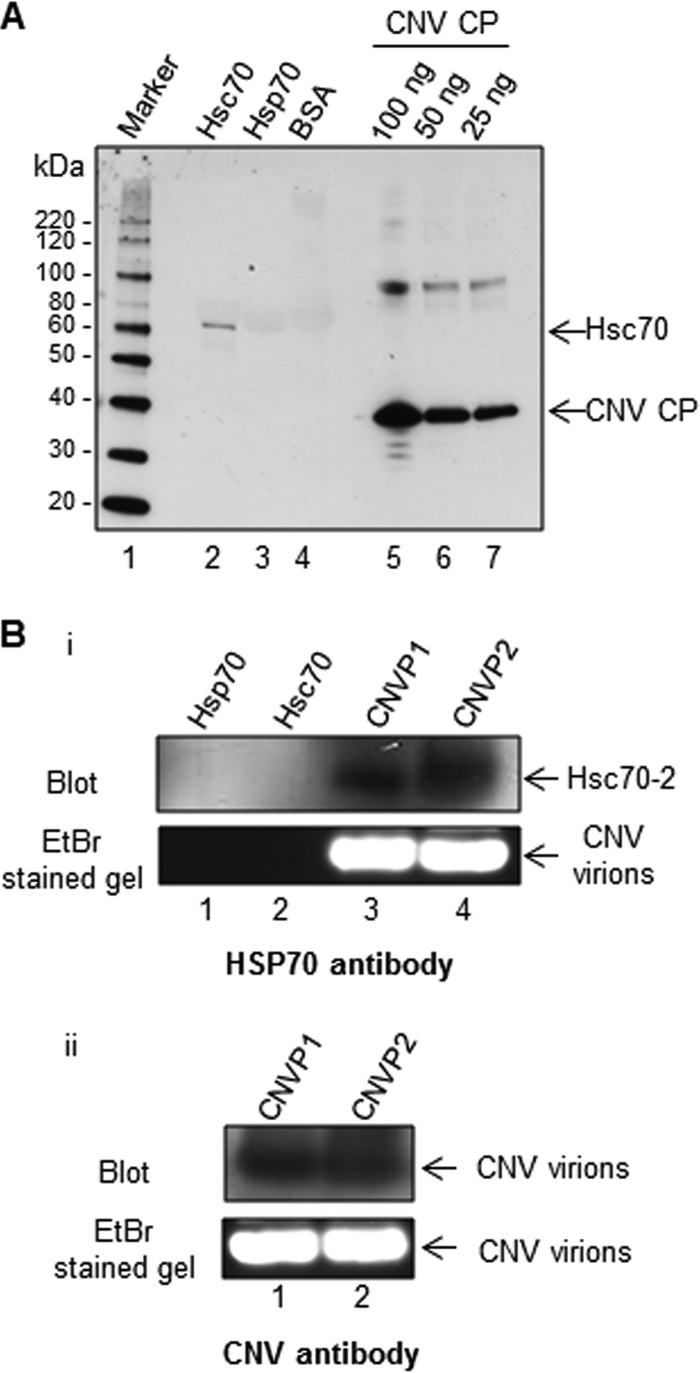
FIG 3.
Purified CNV particles bind to Hsc70 in vitro and in vivo. (A) Virus overlay assay showing that CNV particles bind to Hsc70 in vitro. Equal masses (10 μg) of bovine Hsc70, human Hsp70, and BSA as a negative control along with CNV CP standards (100, 50, and 25 ng, as indicated) were subjected to SDS-PAGE. The proteins were blotted to a nitrocellulose membrane and allowed to renature overnight. The membrane was then incubated with CNV particles, washed, and then probed with a CNV CP-specific antibody. The first lane shows a protein molecular size marker with sizes of protein bands indicated to the left of the blot. (B) HSP70 antibody binds to CNV particles subjected to agarose gel electrophoresis, suggesting a physical interaction between Hsc70 and CNV particles in vivo. Twenty micrograms of two independently purified CNV particle preparations (CNVP1 and CNVP2) were electrophoresed through 1% agarose gel, blotted, and probed with either an HSP70 antibody (panel i) or a CNV antibody made to bacterially expressed S and P domains of the CP as a positive control (panel ii. The upper panel shows the blots, and the lower panel shows the ethidium bromide (EtBr)-stained gels. In panel I, 100 ng of human Hsp70 and bovine Hsc70 was also included in the gel to rule out the possibility that these proteins might comigrate with CNV particles and thus artifactually indicate that CNV particles are bound to Hsc70.