Figure 1.
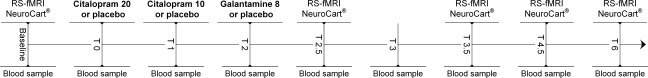
Schematic overview of a study day. Each subject received citalopram, galantamine, and placebo on three different days. At baseline, two RS‐fMRI scan were acquired, followed by the NeuroCart® CNS test battery. After drug administration, four more RS‐fMRI scans were acquired at time points T = 2.5, 3.5, 4.5, and 6 h post dosing, each time followed by the NeuroCart® test battery. During the day, nine blood samples were taken to measure the concentrations of citalopram, desmethylcitalopram, galantamine, cortisol, and prolactin. On each study day there were three moments of administration. The second administration only took place when subjects tolerated the first dose well (did not vomit or feel too nauseous): Galantamine study day: T = 0) placebo T = 1) placebo T = 2) galantamine 8 mg. Citalopram study day: T = 0) citalopram 20 mg T = 1) citalopram 10 mg T = 2) placebo. Placebo study day: T = 0) placebo T = 1) placebo T = 2) placebo.
