Figure 1.
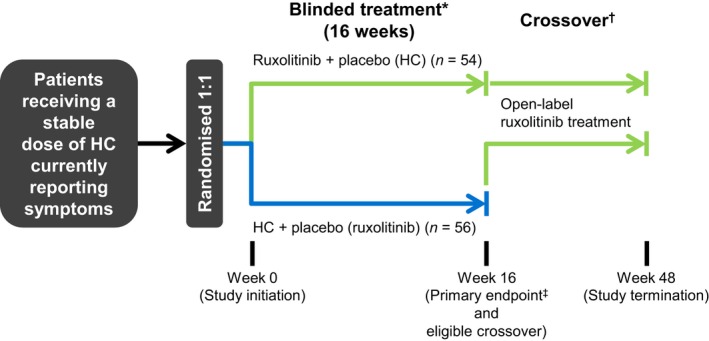
Study design. HC, hydroxycarbamide. *All patients received low‐dose aspirin unless contraindicated. †Patients randomized to HC plus placebo were eligible to cross over after Week 16 to receive open‐label ruxolitinib if safety criteria were met. ‡The primary endpoint was the proportion of patients who achieved ≥50% improvement in Myeloproliferative Neoplasm Symptom Assessment Form total symptom score cytokine symptom cluster at Week 16.
