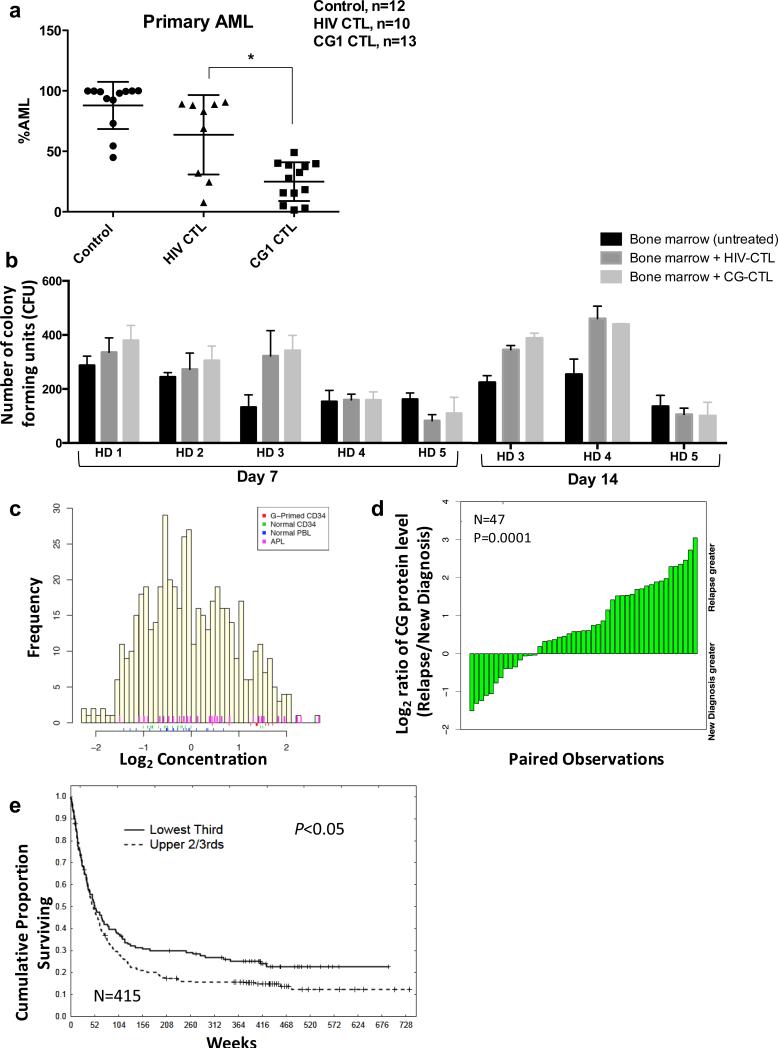
Figure 1.
Cathepsin G is an effective immunotherapeutic target in vivo and is broadly expressed in AML patients. (a) Irradiated NSG mice were injected intravenously with human primary AML blasts (UPN#1; 7 × 106 blasts) on day 0. After confirming leukemia engraftment (~ 3 weeks), mice were treated with either negative control HIV-CTL (0.5 × 106), CG1-CTL (0.5 × 106) or were left untreated. Mice were sacrificed for all groups when any mouse became moribund or during week 7. The results are expressed as percentage of CD33+/CD3− cells from viable hCD45+/mCD45− population within the bone marrow. Results reflect 4 independent experiments; *P<0.01. (b) Bone marrow from 5 healthy donors (HD 1-5) was cultured alone (untreated) or co-cultured with HIV-enriched T cells (HIV-CTL) or CG1-enriched T cells (CG1-CTL) at a 1:5 ratio for 4 hours in cell media. Cells were then resuspended in methylcellulose semi-solid matrix and co-cultured for 7 days. On day 7, colonies were counted and imaged; for HD 3, 4 and 5, colonies were again counted on day 14. Each group was cultured in triplicate and data represent 4 independent experiments. (c) Reverse-phase protein array (RPPA) was used to quantify protein levels of cathepsin G in blasts from 511 newly diagnosed AML patients (yellow bars) and 21 newly diagnosed APL patients (pink bars). Controls included healthy donor CD34+ progenitor cells (n=21, green bars), healthy donor peripheral blood lymphocytes (n=21, blue bars), and GM-CSF-primed healthy donor CD34+ progenitor cells (n=10, red bars). (d) Cathepsin G levels were assessed for 47 patients at diagnosis and relapse and were compared by paired t-test (P=0.0001). (e) Kaplan-Meier plots showing OS in AML patients (n=415) comparing patients with high CG protein expression by RPPA (upper 2/3) to patients with low CG expression (lowest 1/3). Results are significant by Cox univariate model testing (P= 0.04).