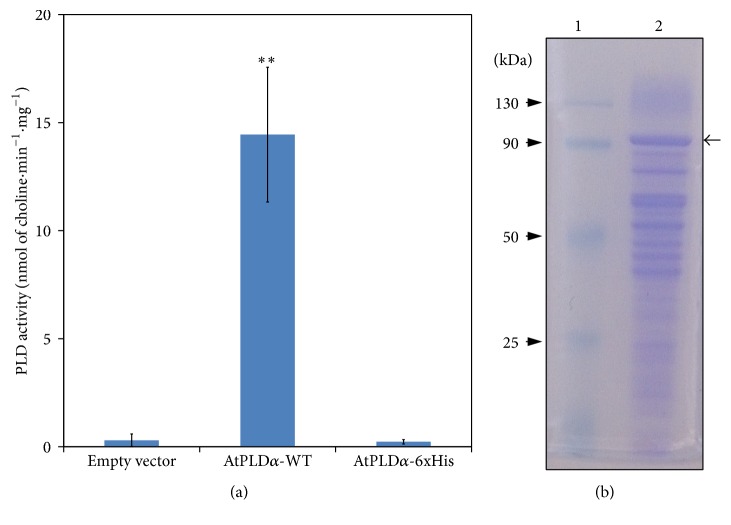
Figure 1.
(a) Recombinant AtPLDα activity in crude protein extracts of Pichia pastoris transformed with the empty vector or expressing either the recombinant AtPLDα-WT or the AtPLDα-6xHis, after 4 days of culture, and using POPC (0.26 mM, final concentration) as the substrate. Values presented are the means ± SD obtained from four independent PCR-positive clones. ∗∗ P < 0.01 (versus blank). (b) SDS-PAGE (10% acrylamide) analysis of the recombinant AtPLDα-WT purified using the purification procedure with octyl-Sepharose CL-4B column (2.5 × 20 cm) (see Materials and Methods). Lane 1: molecular mass marker, lane 2: eluted fraction containing the recombinant AtPLDα-WT. The gel was stained with Coomassie Brilliant Blue R-250 to reveal the proteins. The arrow on the right indicates the position of the AtPLDα-WT.