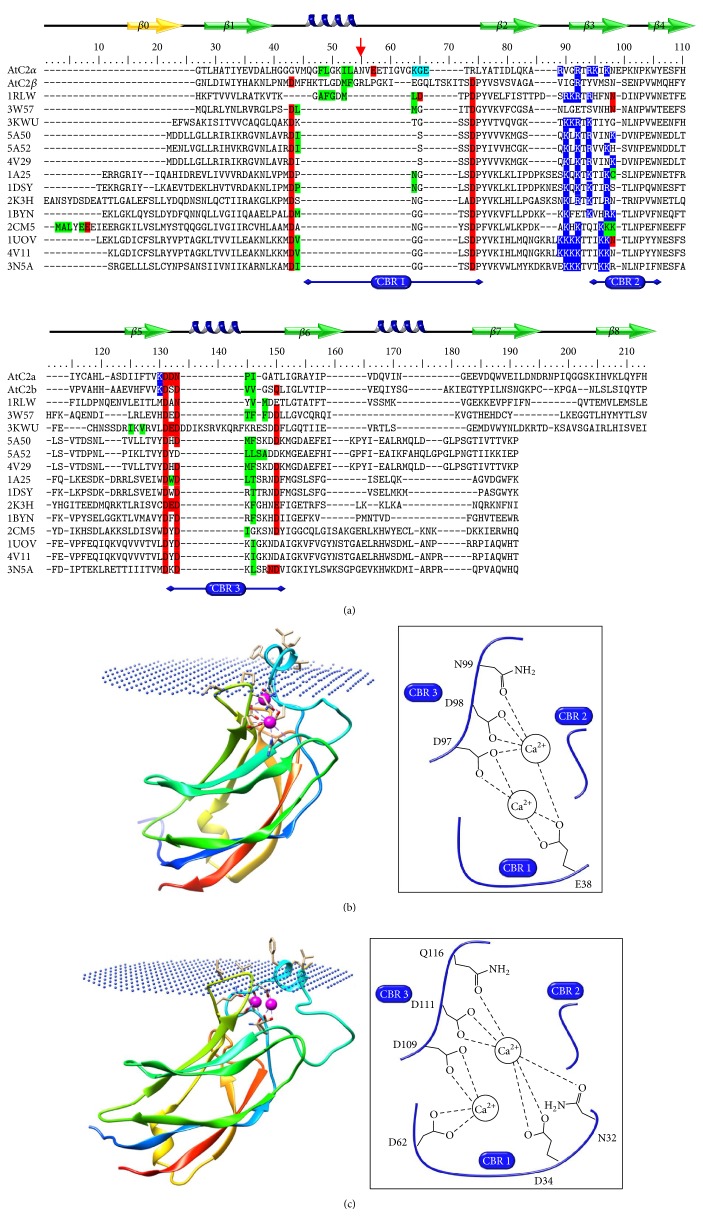
Figure 6.
(a) Multiple sequence alignment of C2 domains. Amino acid sequences of C2α and C2β were aligned with various known C2 domain structures (see Materials and Methods). Residues highlighted in red represent Ca2+ binding residues extracted from structures and residues highlighted in green represent membrane binding residues extracted from the OPM database. The proposed α-helix between β1 and β2 strands is indicated in blue. CBR: Ca2+ binding region. Strand β0, colored in yellow, corresponds to the first β strand present in typology I. The observed cleavage on the recombinant AtPLDα is indicated by the arrow (red). The PDB entries correspond to cPLA2 (1RLW), perforin (3W57), Munc13-1 (3KWU), Car4 (5A50 and 4V29), Car4 (5A52), PKCα (1DSY), PKCβ (1A25), rabphilin-3A (2K3H and 2CM5), Synaptotagmin-1 C2A (1BYN), and C2B (1UOV), SV2A (4V11), and Synaptotagmin-7 C2B (3N5A). Modeled structures of the C2α domain (b) and the C2β domain (c), using the I-tasser server and docked to membrane (dummy) using the PPM server. Ca2+ is colored in pink.