Figure 2.
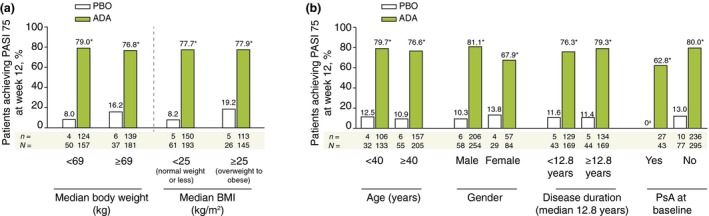
PASI 75 Response at week 12 for Patient Subgroups (ITT_A Population). (a) *P < 0.001. 95% CIs: median bodyweight (kg) <69 [61.1, 80.8] and ≥69 [47.2, 74.0]; median BMI (kg/m2) <25 [60.5, 78.6] and ≥25 [42.1, 75.3]. Non‐responder imputation. (b) *P < 0.001. 95% CIs: age (years) <40 [53.9, 80.5] and ≥40 [55.6, 75.8]; male [61.6, 80.0] and female [38.0, 70.1]; median duration of psoriasis (years) <12.8 [53.2, 76.2] and ≥12.8 [56.7, 79.1]; psoriatic arthritis at baseline, yes [48.3, 77.2] and no [58.2, 75.8]. Non‐responder imputation. a N = 10. ADA, adalimumab 40 mg every‐other‐week dosing; BMI, body mass index; CI, confidence intervals; PASI, Psoriasis Area Severity Index; PBO, placebo; PsA, psoriatic arthritis.
