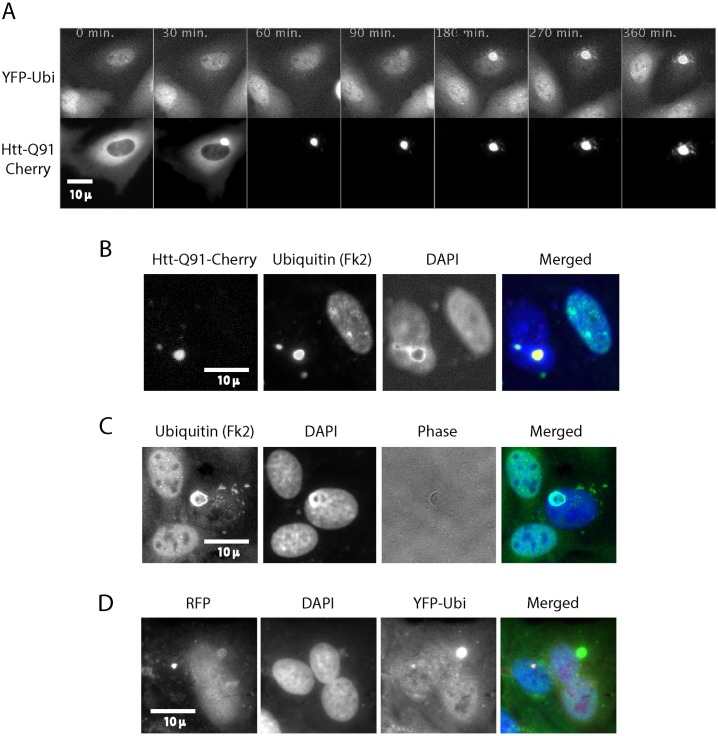
Fig 1. Poly Q aggregates accumulate ubiquitin and lead to depletion of nuclear ubiquitin.
Cells stably expressing YFP-Ubi were transiently transfected with Htt-Q91-Cherry and followed by live cell imaging (S3 Mov). Shortly after the Htt-Q91-Cherry aggregates it starts to accumulate ubiquitin. T = 0 was arbitrarily set to the time protein aggregation started in this particular cell. Strikingly YFP-Ubi staining in the nucleus is depleted (A). U2OS Cells were transiently transfected with Htt-Q91-Cherry and fixed for immunofluorescence with an antibody that identifies ubiquitinated proteins (Fk2). The experiment was repeated in N2A and PC12 cells (S2 Fig). In each of these lines the cells with the IB lack nuclear ubiquitin staining compared to the non-expressing cells. (B). U2OS Cells were transiently transfected with untagged Htt-Q91 and fixed for immunofluorescence with Fk2. Ubiquitin accumulated on the untagged IB and was depleted from the nucleus like with the Htt-Q91-Cherry (C). U2OS cells were co-transfected with Ubi[9]-mRFP[1] and YFP-Htt-Q91. Overexpression of ubiquitin reduces depletion of nuclear ubiquitin by Htt-Q91 in spite of the accumulation of ubiquitin on the IB (D).