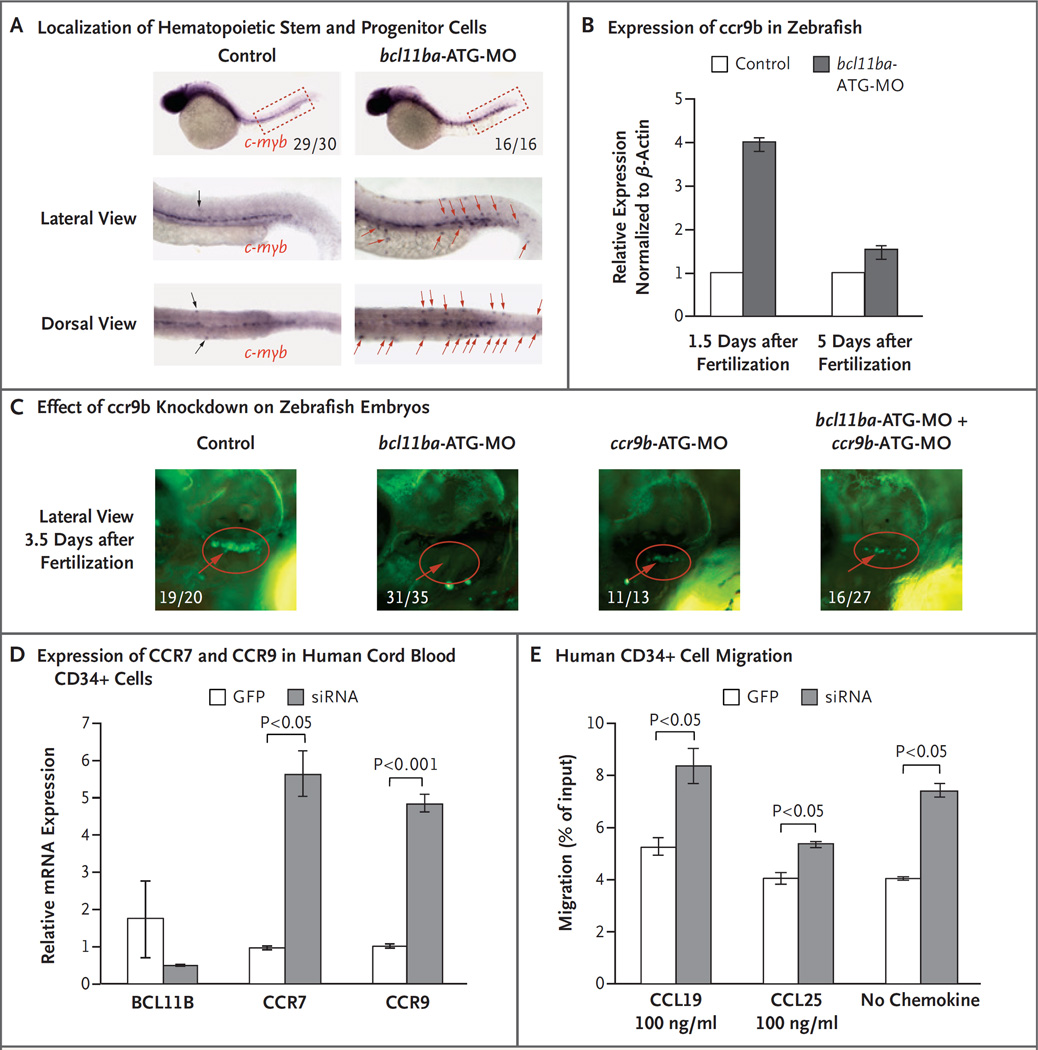
Figure 4. Role of BCL11B in Migration of Hematopoietic Progenitor Cells in Zebrafish and Humans.
Panel A shows c-myb WISH staining at 36 hours (1.5 days) after fertilization to examine the localization of hematopoietic stem and progenitor cells. The fractions indicate the numbers of zebrafish that had the depicted phenotype out of the total number examined. Displaced hematopoietic stem and progenitor cells are marked by black (control morpholino oligonucleotide [MO] injected) and red (bcl11ba-MO injected) arrows. Panel B shows the relative ccr9b expression, measured by quantitative PCR in control embryos and bcl11ba morphants 1.5 and 5 days after fertilization. Expression in triplicate samples was quantified, normalized to that of β-actin and depicted as mean fold change (the expression in experimental embryos relative to the expression in control-injected embryos, which was defined as 1); I bars indicate standard deviations. Panel C shows the effect of morpholino (ccr9b-ATG-MO) knockdown of ccr9b, either alone or in combination with bcl11ba-ATG-MO, on thymic seeding at 3.5 days after fertilization in transgenic cd41:GFP zebrafish embryos (red circles). Numbers are the fraction of embryos that had the depicted phenotype. Panel D shows the expression, normalized to that of housekeeping gene GAPDH, of BCL11B, CCR7, and CCR9 mRNA in human cord-blood CD34+ cells transduced with lentivirus encoding GFP alone or BCL11B siRNA–GFP. Panel E shows the in vitro transwell migration of human CD34+ cells, transduced as in Panel C, in response to CCL19 or CCL25 (100 ng per milliliter) or no chemokine.