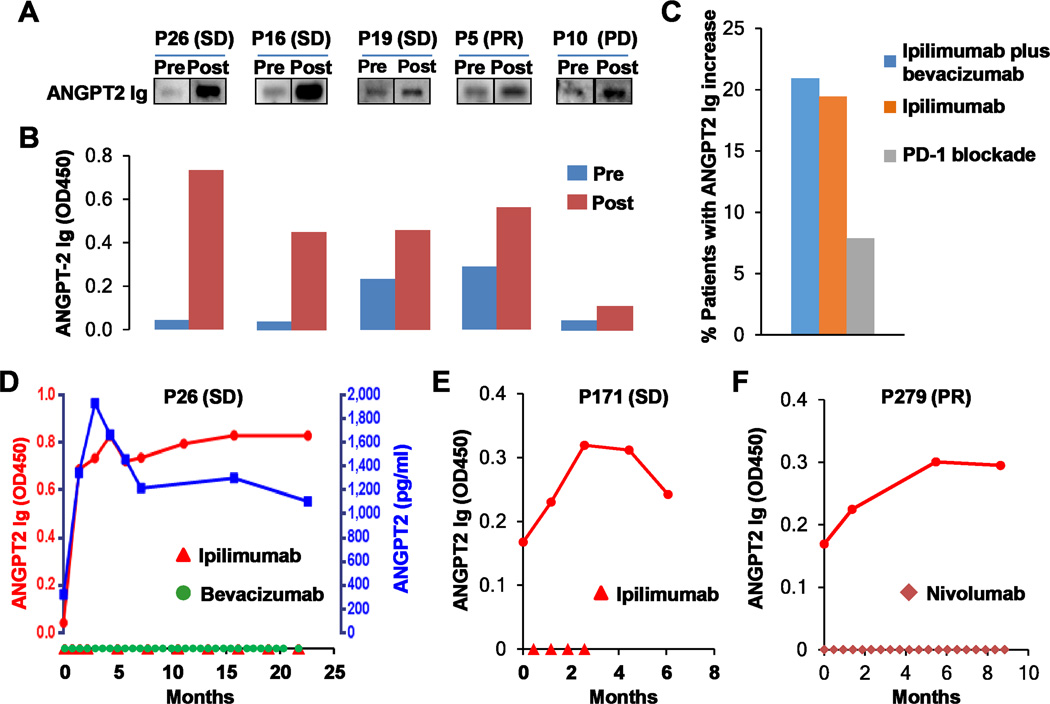
Fig. 6.
Immune checkpoint therapy elicited antibody responses to ANGPT2. A and B, ANGPT2 antibodies were detected in pre- and posttreatment plasma samples of ipilimumab plus bevacizumab-treated patients by immunoblot analysis (A) and ELISA (B). Clinical responses are also indicated. C, Proportions of patients receiving ipilimumab plus bevacizumab (n =43), ipilimumab (n = 36), and PD-1 blockade (n = 38) displayed an increase by 40% or more in ANGPT2 antibody concentrations. D–F, Longitudinal analysis of serum ANGPT2 and/or ANGPT2 antibodies in patients receiving ipilimumab plus bevacizumab (D), ipilimumab (E), or PD-1 blockade (F). Dosing of ipilimumab, bevacizumab, or nivolumab was indicated on the x-axis. Day 0 is pretreatment.