Fig. 1.
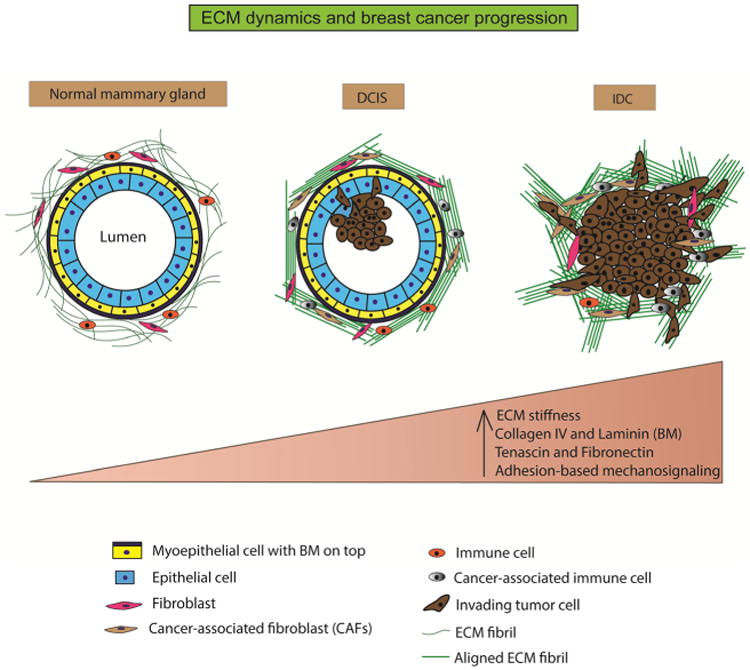
ECM dynamics and breast cancer progression. The normal mammary gland is characterized by a well-defined layer of epithelial cells around a central lumen. It is surrounded by a layer of contractile myoepithelial cells, which, in turn, is encased by the basement membrane. The interstitial matrix or the stroma surrounds this structure and comprises of randomly organized fibrillar collagen. The stroma also hosts fibroblasts and immune cells that function in ECM maintenance, immune surveillance, and mammary homeostasis. During ductal carcinoma in situ (DCIS), the epithelial cells undergo unregulated proliferation and start infiltrating into the central lumen. The ECM fibrils (collagen) are cross-linked and organized in bundles parallel to the tumor boundary. Stromal composition is altered with the appearance of cancer-associated fibroblasts and immune cells. During invasive ductal carcinoma (IDC), the lumen is almost completely filled with epithelial cancer cells. The ECM fibrils undergo further cross-linking and organized themselves perpendicular to the tumor boundary to provide migration tracks for the tumor cells to invade into neighboring tissue and blood vessels. Overall, the progression from normal mammary tissue to IDC is accompanied by increasing ECM stiffness, altered ECM composition, and aberrant mechanosignaling
