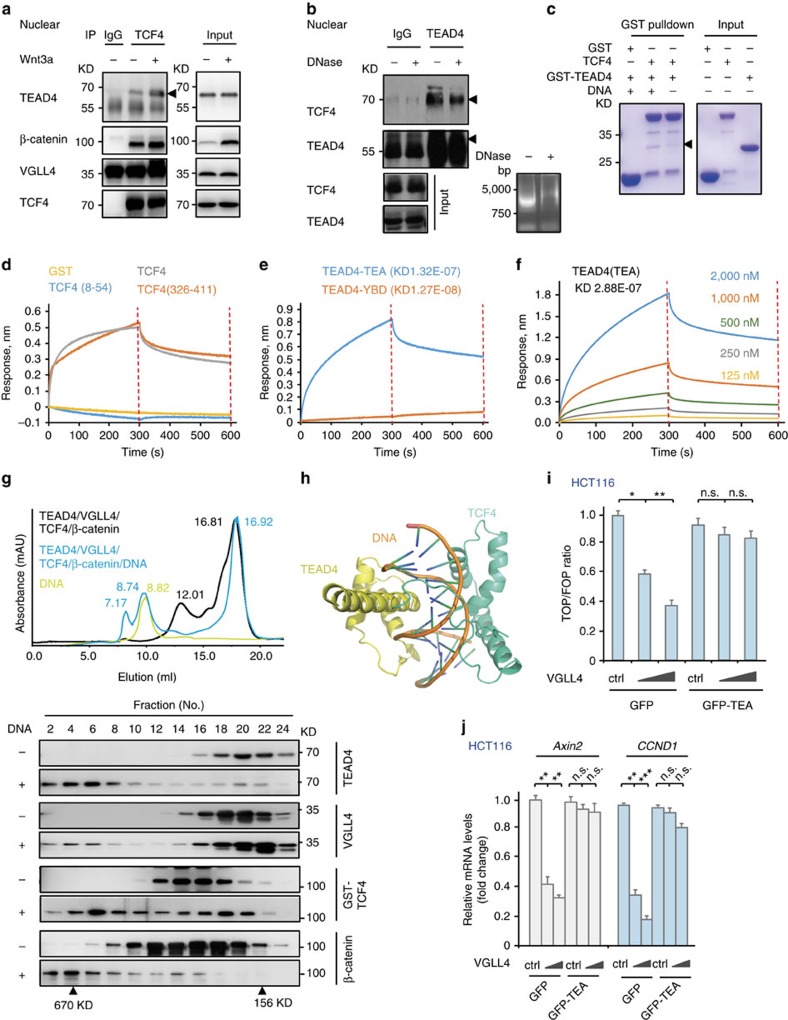
Figure 5. TEAD4 and TCF4 forms a complex for to cobind target genes.
(a) Coimmunoprecipitation to analyse the association of TEAD4 and TCF4. HEK293T cells were treated with or without Wnt3a for 4 h. Then the cell lysates were incubated and immunoprecipitated with anti-TCF4 antibody or control IgG. (b) Co-IP assay to determine the interaction of TEAD4 and TCF4 with or without DNAse. Digestion of DNA was monitored by agarose gel. (c) Pulldown assay to detect the TEAD4-TCF4 interaction using purified recombinant proteins with or without a DNA fragment of SOX2 promoter. (d–f) Biolayer Interferometry analysis of interactions between TCF4 and TEAD4 using purified recombinant proteins. (d) Full length TEAD4 (coated on chip) can bind to GST-tagged full length TCF4 or GST-tagged TCF4 (amino acids 326–411), but not GST-tagged TCF (amino acids 8–54) or GST. (e) Full length TCF4 (coated on chip) can bind to the TEA domain but not the YBD of TEAD4. (f) TCF4 (amino acids 326–411) (coated on chip) can bind to the TEA domain of TEAD4 in a dose-dependent manner. (g) Gel filtration analysis of interactions among VGLL4, TEAD4, TCF4 and β-catenin with or without the SOX2 DNA. (h) Structure modelling of the interaction of TCF4 in complex with TEAD4 and DNA. (i,j) Overexpression of the TEAD4 TEA domain abrogated VGLL4-mediated inhibition of TOP-FLASH reporter activity (i) and transcription of Wnt target genes (j) in HCT116 cells. Data in i and j represent the mean±s.d. from one experiment. Experiments we repeated three times. Unpaired t tests were used to compare the difference between groups. *P<0.05, **P<0.01, ***P<0.001, significant relative to control. n.s., no statistics significance.