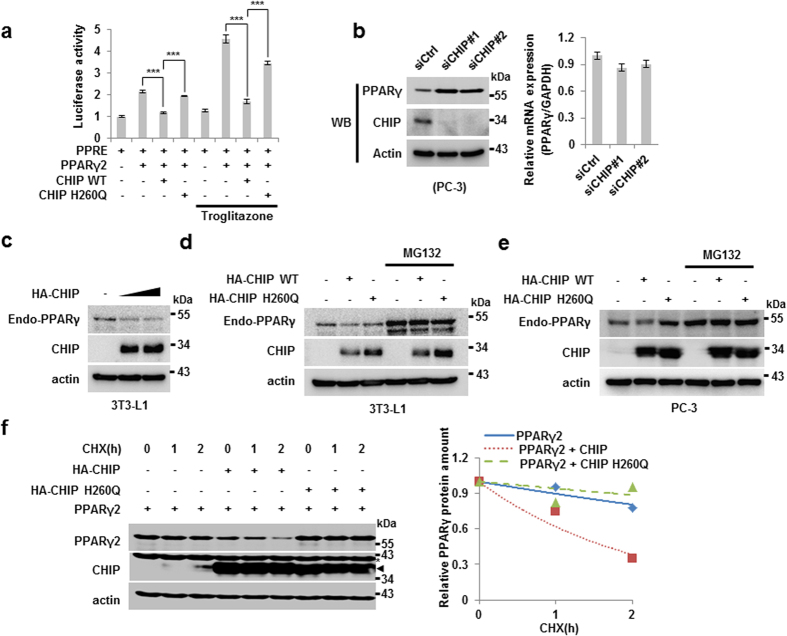
Figure 2. CHIP degrades PPARγ protein through the proteasomal pathway via an E3-ligase function.
(a) CHIP represses the transcriptional activity of PPARγ. H1299 cells were transfected with PPRE, pcDNA3.1-PPARγ2, pcDNA3-FLAG-CHIP, and pcDNA3-FLAG-CHIP H260Q plasmids with or without troglitazone. Cells were measured by luminometer. Data are presented means ± SD; n = 3; **P < 0.01, and ***P < 0.001 compared to each lane. (b) The CHIP protein level was increased by CHIP knockdown using siRNAs. Western blots of lysates of PC-3 cells transfected with the indicated plasmids using siRNA IMAX were performed using the indicated antibodies. (c) Endogenous PPARγ protein was degraded by CHIP. Preadipocyte, 3T3-L1 cells were treated with DMI cocktail for 2 days, were transfected with pcDNA3-HA-CHIP plasmid dose dependent manner. Indicated protein was measured by western blotting. (d,e) CHIP degrades PPARγ protein through the E3-ligase function and proteasomal manner. (d) DMI treated 3T3-L1 cells were transfected with HA-CHIP and HA-CHIP H260Q mutant expressing vectors with or without MG132. Each protein was detected by western blotting indicated antibodies. (e) PC-3 cells were transfected with pcDNA3-HA-CHIP and pcDNA3-HA-CHIP H260Q mutant with or without MG132. Lyzed cells were analyzed by western blotting indicated antibodies. (f) The PPARγ protein was destabilized by wild-type CHIP but not by the H260Q mutant. Western blots of H1299 cells transfected with plasmids expressing PPARγ, FLAG-CHIP, and FLAG-CHIP H260Q in the presence or absence of 50 μg/mL CHX (cycloheximide) treatment were performed using the indicated antibodies and were measured with the Image J program. Asterisk indicates an actin band.