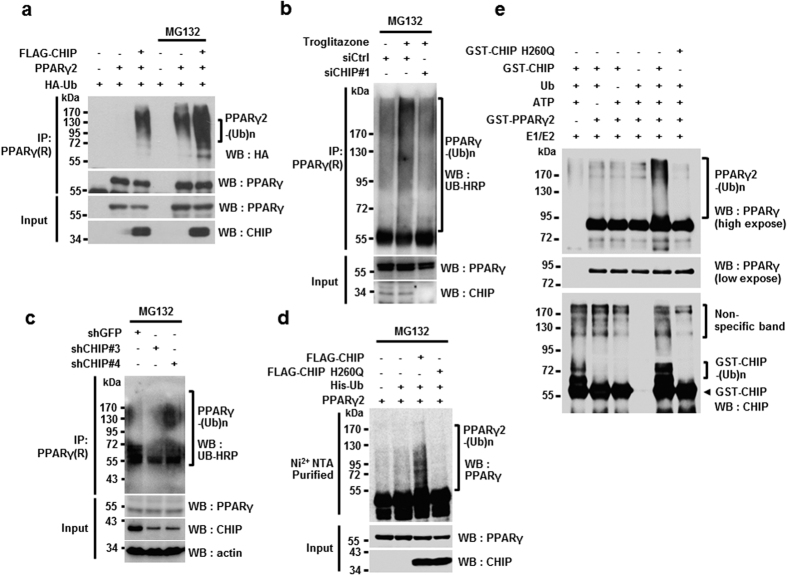
Figure 3. CHIP mediates ubiquitylation of PPARγ2 through its E3 ligase activity.
(a) CHIP induces ubiquitylation of PPARγ2. Western blots of H1299 cells transfected with pcDNA3.1-PPARγ, pcDNA3.1-FLAG-CHIP, and PMH-HA-Ub with or without MG132 were performed using the indicated antibodies. (b,c) Elimination of CHIP did not increase the ubiquitylation of PPARγ. Lysates of PC-3 cells transfected with siRNA control (siCtrl) or siRNA CHIP #1 and treated with MG132 were immunoprecipitated with polyclonal PPARγ antibodies and western blots were performed using the indicated antibodies. (b) Lysates of 3T3-L1 cells transfected with CHIP knockdown plasmids expressing shCHIP #3 and shCHIP #4 or control plasmid (shGFP), differentiated by DMI treatment, and treated with MG132 were immunoprecipitated using polyclonal PPARγ. (c) Western blots were performed on the above lysates using the indicated antibodies. (d) PPARγ2 protein was ubiquitylated by wild-type CHIP but not the H260Q mutant. Western blots of H1299 cells transfected with asdescribe above all, with His-ubiquitin-conjugated proteins pulled down using Ni2+-NTA beads. The indicated antibodies were used. (e) CHIP directly ubiquitinates PPARγ2 in vitro. Western blots of purified GST-PPARγ2 recombinant protein incubated with ATP, E1, E2, and ubiquitin in the presence or absence of wild-type GST-CHIP or GST-CHIP H260Q mutant were performed using the indicated antibodies.