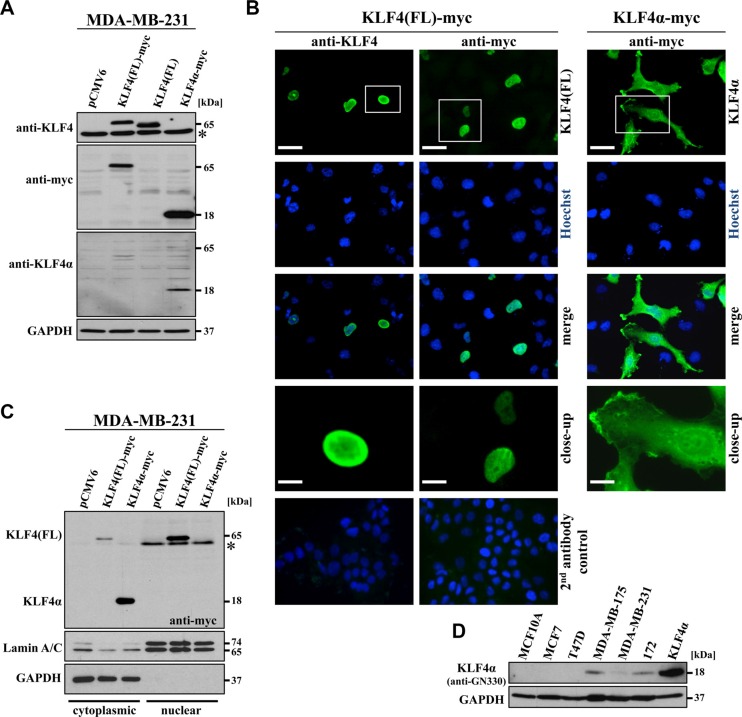
Figure 3. Characterization of KLF4α expression.
(A) MDA-MB-231 cells were transfected with the indicated plasmids and immunoblotted with α-KLF4, which only detects KLF4(FL), with α-myc, which detects both myc-tagged variants of KLF4, and with α-GN330, which specifically recognizes KLF4α. *background band. (B) Immunofluorescent stainings of transfected MDA-MB-231 cells 24 h post-transfection show nuclear staining for KLF4(FL) and cytoplasmic staining for KLF4α. Hoechst stain was used for the visualization of nuclei. Scale bar: 50 μm (top); 10 μm (close-up). The bottom row indicates the secondary antibody only controls merged with the respective Hoechst staining (left side: goat-anti-rabbit IgG-FITC; right side: goat-anti-mouse IgG-FITC). (C) Transfected MDA-MB-231 cells were extracted for the cytoplasmic and the nuclear fractions 36 hours after transfection and immunoblotted. KLF4α is only found in the cytoplasmic, GAPDH-positive fraction, whereas most of KLF4(FL) is present in the nuclear, LaminA/C-positive fraction. * background band. (D) Western blot analysis of protein extracts of the normal breast cell line (MCF10A), four breast cancer cell lines as well as a p53-mutant Li Fraumeni cell line (172) in comparison to KLF4α-transfected MDA-MB-231 cells for KLF4α levels. Note that endogenous KLF4α (∼18kDa) is detectable in MDA-MB-175, MDA-MB-231, and 172 cells. GAPDH: glyceraldehyde-3-phosphate dehydrogenase.