Figure 10. Schematic of signaling pathways involved in MA-mediated ER stress in astrocytes.
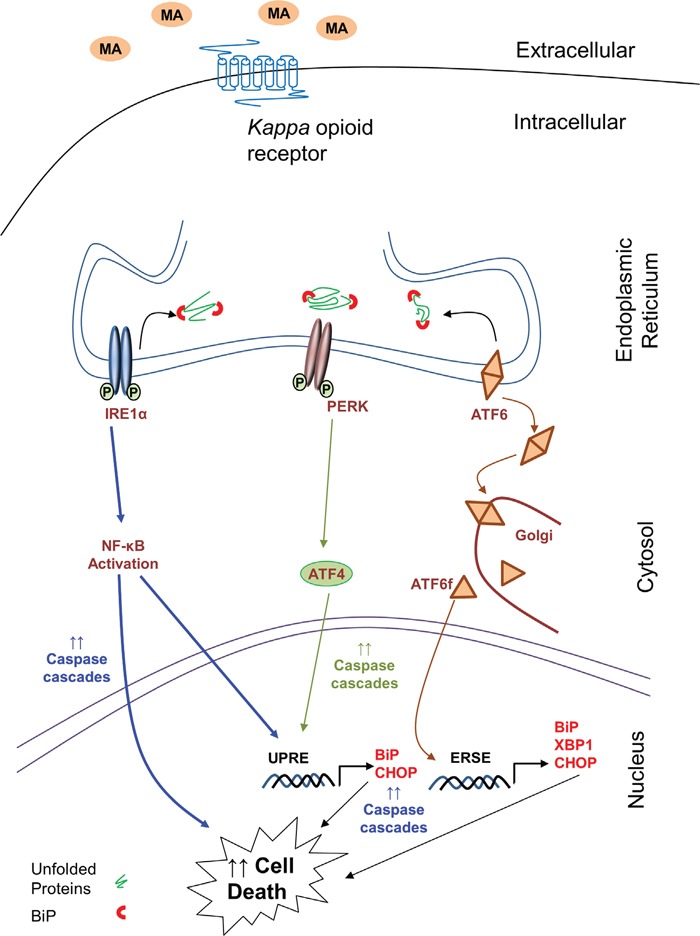
The signaling pathway sought in the present work is ER stress signaling cascade, which is responsible for MA-mediated cell death in astrocytes. The increase in the levels of BiP and CHOP suggest increased ER stress. This results in activation of ATF6, IRE1α and PERK pathways. Activation of ATF6 is signified by increased accumulation of ATF6 in golgi, where it is cleaved. The free cytosolic form of ATF6 then increases the transcription of BiP, CHOP and XBP1 via ER stress-response element (ERSE). Activation of IRE1α leads to activation of NF-κB, which further increases transcription of CHOP and engage various caspases to signal for apoptosis. Similarly, activation of PERK leads to increase in ATF4, which can increased transcription of BiP and CHOP via unfolded protein response element (UPRE). Finally, the convergent effect of these three mechanisms further accumulates CHOP leading to apoptosis via caspase cascades. Thus increase in ER stress due to MA activates the intrinsic caspase cascade via caspase-3 and caspase-9 cleavage. The kappa opioid receptor serves as an anchor for MA, which allows for the downstream activation of various events in the ER stress pathways. Finally, these cascades lead to cell death in astrocytes.
