Figure 6. AR-mediated anoikis of CTCs might be due to cytoskeletal rearrangement.
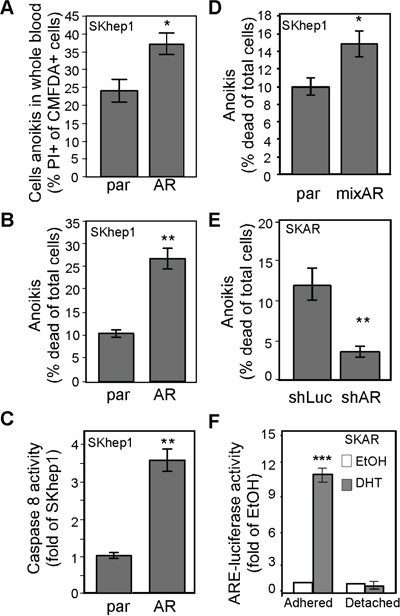
A. AR promoted HCC cell anoikis in whole blood. The parental (par) and SKhep1 cells (AR) were pre-incubated with CMFDA, mixed with whole blood, and then further incubated in non-adhesion conditions to measure cell anoikis. The percentage of CMFDA double-positive cells was higher in AR cells than in par cells. B. AR promoted anoikis in regular culture medium. Cells were incubated in DMEM in adhesion conditions, and cell death was measured with PI (propidium iodine; 5 μM). The percentage of cells exhibiting anoikis was higher in AR cells than in par cells. C. AR overexpression resulted in increased Caspase 8 activity in non-adhesion cells. D. Anoikis was promoted in mixed AR stable clones but not in parental cells. E. AR knockdown resulted in reduced anoikis. SKAR cells were infected with AR-specific (shAR) or luciferase-specific (shLuc) shRNA. Anoikis was detected as described above. The percentage of cells exhibiting anoikis was lower in shAR-infected cells than in shLuc-infected cells. F. ARE transactivation activity was abolished in non-adhesion conditions. The SKAR cells were co-transfected with ARE-luciferase and pRL-TK (transfection control) constructs, treated with EtOH or 10 nM DHT, and then analyzed for dual-luciferase activity in either adhesion (p<0.0001) or non-adhesion conditions. ARE-luciferase activity dramatically increased in DHT-treated cells in adhesion conditions but not in non-adhesion conditions. The plotted data were from the mean value of at least three independent experiments, and SD was used to show the variation in the experiments. A single * label represents significance at p < 0.05, and a double * label represents significance at p < 0.01.
