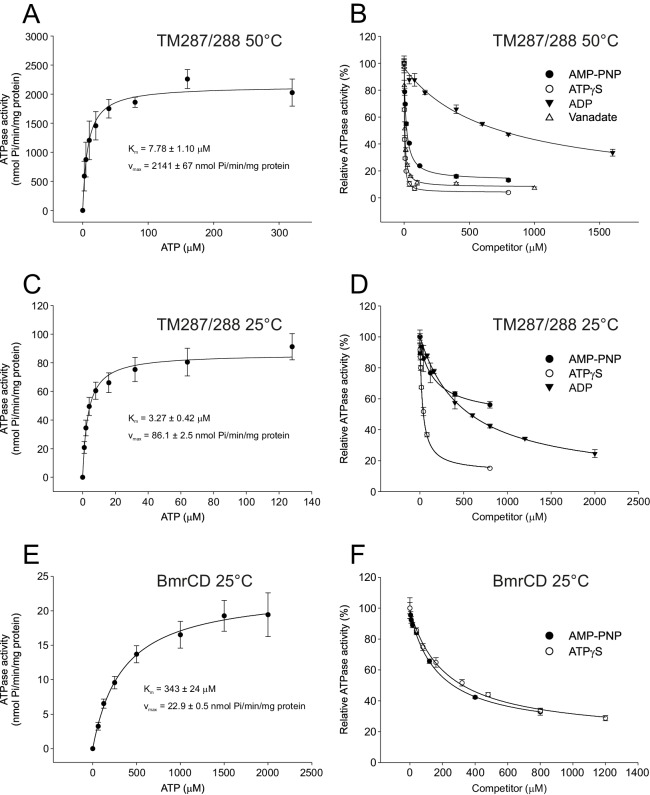
Figure 3. Inhibition of ATPase activity of TM287/288 and BmrCD by vanadate and nucleotides.
Km and vmax values for ATP hydrolysis by TM287/288 at 50°C (A) and 25°C (C) and BmrCD at 25°C (E) were determined by measuring ATPase activities at increasing ATP concentrations. Inhibition of ATP hydrolysis of TM287/288 was determined in the presence of increasing concentrations of vanadate at 50°C (B) or AMP-PNP, ATPγS and ADP at 50°C (B) or 25°C (D). Inhibition of ATP hydrolysis of BmrCD by AMP-PNP and ATPγS was determined at 25°C (F). In the inhibition assays, 500 µM and 2500 µM ATP were used for TM287/288 and BmrCD, respectively. The curves were fitted with a hyperbolic decay function to obtain IC50 values, which were used together with the corresponding Km to calculate Ki (Table 2). The error bars of the measurement points are standard deviations of three technical replicates.