Abstract
The influence of extraction temperature, powder concentration, and extraction time on the antioxidant properties of aqueous ginger extract was investigated. The possibility of estimating the antioxidant properties of the extract from its absorbance and colour properties was also investigated. Results indicated that powder concentration was the most significant factor to consider in optimizing antioxidant extraction. However, temperature and time still influenced the 2,2′-azinobis-(3-ethylbenzothiazoline-6-sulfonic acid) (ABTS) radical scavenging activity while extraction temperature influenced the 2,2-diphenyl-1-picrylhydrazyl (DPPH) radical scavenging activity of the extract. Using the total phenol content, total flavonoid content, ABTS radical scavenging activity, and DPPH radical scavenging activity of the extract, the multiresponse optimization condition for extraction of antioxidant based on the experimental range studied is 96°C, 2.10 g/100 mL, and 90 min. The absorbance of the ginger extract at 610 nm could be exploited for rapid estimation of its total flavonoid and polyphenol with a R2 of 0.713 and 0.753, respectively.
Keywords: ginger, aqueous extraction, antioxidants, response surface, multivariate regression
INTRODUCTION
Ginger is a common dietary adjunct that contributes to the taste and flavour of foods, and is also an important traditional Chinese medicine (1). Ginger also has high antioxidant potential (2). Cell culture studies also showed that ginger has antioxidant properties (3). The major phytochemicals in ginger include gingerols and shogaols. Both gingerols and shogaols exhibit a host of biological activities, ranging from anticancer, antioxidant, antimicrobial, anti-inflammatory, and anti-allergic to various central nervous system activities (4). Processing conditions can affect extraction of phytochemicals from plants (5). The water extract of ginger has been reported to inhibit human low-density lipoprotein oxidation in vitro (6). Inclusion of ginger or ginger extracts in nutraceutical formulations could provide valuable protection against diabetes, cardiac, and hepatic disorders (4). An increase in phytochemical concentration has been linked to increase in antioxidant activities of plant extract; however, there is a point where at very high concentration of these phytochemicals they may begin to act as pro-oxidant (7). Therefore it may be more reasonable to focus on maximizing the antioxidant activity of the extract rather than just maximising the concentration of the phytochemicals. Thus in this study we sought to simultaneously maximize the antioxidant property of aqueous ginger extract. We also investigated the possibility of developing rapid protocols that could be used for estimating some antioxidant properties of aqueous ginger extracts.
MATERIALS AND METHODS
Plant material and processing
Ginger rhizomes were procured from Kaduna State in Nigeria. The ginger rhizomes were peeled, sundried, and ground. The powder sample was passed through a 1.4 mm sieve. The obtained powder was wrapped in aluminium foil and stored under refrigerated condition (4°C) for subsequent analysis.
Extraction
The aqueous extract was obtained as described by Makanjuola et al. (5). The extraction was done in a conical flask placed on temperature controlled magnetic stirrer (UC 152, Bibby Scientific Ltd., Stone, UK). The stirrer speed was set at scale 3. Water was introduced into a conical flask. The flask was covered with aluminium foil to protect from light. To ensure the accuracy of the extraction temperature, a temperature controller (SCT 1, Bibby Scientific Ltd.) was placed inside the flask and connected to the temperature controlled magnetic stirrer. Once the required extraction temperature was reached, the required weight of ginger powder was introduced into the conical flask. The extraction was continued until the required time was reached. The extract was then filtered to remove the residue.
Response surface methodology
The response surface modelling was carried out as described by Makanjuola et al. (5). A face centered central composite design was used. The design consisted of 20 experimental runs: 8 factorial points, 6 axial points, and 6 central points. The range of the independent variables investigated were extraction temperature (TEM: 30~ 96°C), powder to solvent ratio (CON: 0.12~2.10 g/100 mL), and extraction time (TIM: 5~90 min). The response variables were antioxidant properties of the extracts. The antioxidant properties were total flavonoid content (TFC), total phenol content (TPC), 2,2′-azinobis-(3-ethylbenzothiazoline-6-sulfonic acid) (ABTS) radical activity, 2,2-diphenyl-1-picrylhydrazyl (DPPH) radical activity, peroxide scavenging activity, and iron chelating activity. Data were fitted to different models. Response surface methodology models considered were linear, 2 factor interaction, and quadratic. Analysis of variance (ANOVA) was carried out to choose the best model. The best model that was chosen was further subjected to backward regression to remove redundant variables. Both single response and multi-response optimization were conducted, using the desirability concept. The optimization was set to maximize all the antioxidant properties and the process conditions were set to be within the experimental range. The antioxidant properties were all given an equal weighting of 1 for the optimization. The quality of the model was evaluated using the lack-of-fit, the coefficient of determination (R2), adjusted R2, predicted R2, and adequate precision.
Prediction of antioxidant properties from colour and absorbance property of the extracts
The colour (CIE L*, a*, and b*) and sample absorbance at 510 nm (A510) and 610 nm (A610) of the extracts were measured. Using the a* and b* values, the hue and chroma of the extracts were calculated. The hue index value was also estimated using the values of A510 and A610. Hue index has been used in the caramel industry as an indicator of its colour (8). The suitability of hue index in evaluating colour of tea has also been reported (9). A multivariate regression analysis was carried out on the obtained data as described by Makanjuola et al. (5). The dependent variables were the antioxidant properties. The independent variables were L*, a*, b*, hue, chroma, A510, A610, A510/A610, and hue index. The multivariate statistics employed were ordinary least square regression (OLSR), principal component regression (PCR) and partial least square regression (PLSR). The data were scaled and centered before running the regression analysis. In the PCR analysis, the regression was run for components that explained between 90 to 99% of the variation in the independent variables. The dependent variables were also subjected to transformations (log10, square root and inverse square root) to check for improvement in the model quality.
Colour and hue index analysis
Colour (L*, a*, and b*) was measured with a spectrophotometer CM-700d (Konica Minolta Sensing, Osaka, Japan) that was calibrated against a white plate. Hue was calculated as θ using eq. (1).
| (1) |
The following transformations were applied to the calculated θ (10);
| (2) |
| (3) |
| (4) |
| (5) |
Chroma was calculated with eq. 6.
| (6) |
The hue index was calculated from eq. 7.
| (7) |
The A610 and A510 values were obtained by measuring the absorbance of the extract against a distilled water blank in a spectrophotometer.
Determination of antioxidant properties
TPC, TFC, ABTS radical scavenging ability, peroxide scavenging activity, iron chelating activity, and DPPH radical scavenging activity were assayed as described by Makanjuola et al. (5).
Software
The response surface analysis was done using Design Expert v 7.0.0 (Stat-Ease, Inc., Minneapolis, MN, USA). The multivariate statistics was carried out with XLSTAT Pro, 2013 (Addinsoft, New York, NY, USA).
RESULTS AND DISCUSSION
CON had a significant effect (P<0.05) on the TFC and TPC of the aqueous ginger extracts (Table 1). The ABTS activity of the aqueous ginger extract was influenced (P<0.05) by TEM, CON, TIM, the interaction between CON and TIM, and the quadratic effects of CON, TEM, and TIM. TEM, CON, the interaction between TEM and CON, and quadratic effect of CON all had significant influence (P<0.005) on the DPPH radical scavenging activity of the aqueous ginger extracts.
Table 1.
Response surface model for aqueous extraction of ginger powder
| Source | TFC | TPC | ABTS | Peroxide scavenging activity | Iron chelating activity | DPPH |
|---|---|---|---|---|---|---|
| Transformation | (TFC+28.00)0.34 | na | 1.0/Sqrt | (DPPH+19) | ||
| Intercept | 3.9974 | 16.8173 | 1.0744 | 73.4920 | 65.3010 | 83.4637 |
| TEM | 0.0161 | −2.3704 | ||||
| CON | 4.2744 | 82.6237 | −1.0432 | −7.5812 | ||
| TIM | 8.6137E−3 | |||||
| TEM×CON | 0.3080 | |||||
| TEM×TIM | ||||||
| CON×TIM | −1.4728E–3 | |||||
| TEM2 | −1.2901E–4 | 0.0203 | ||||
| CON2 | 0.4261 | |||||
| TIM2 | −6.9939E–5 | |||||
| Model (P-value) | <0.0001 | <0.0001 | 0.0003 | 0.0008 | ||
| Lack of Fit | 0.6835 | 0.8919 | 0.0714 | 0.0093 | 0.0102 | 0.5088 |
| R2 | 0.6329 | 0.6214 | 0.8613 | 0 | 0 | 0.6974 |
| Adj R2 | 0.6125 | 0.6004 | 0.7804 | 0 | 0 | 0.6167 |
| Pred R2 | 0.5723 | 0.5424 | 0.5400 | −0.1080 | −0.1080 | 0.4073 |
| Adeq Prec | 11.142 | 10.871 | 11.144 | 9.811 |
TFC, total flavonoid content; TPC, total phenol content; ABTS, 2,2′-azinobis-(3-ethylbenzothiazoline-6-sulfonic acid) radical scavenging activity; DPPH, 2,2-diphenyl-1-picrylhydrazyl radical scavenging activity.
TEM, temperature; CON, concentration; TIM, time; Adj R2, adjusted R2; Pred R2, predicted R2; Adeq Prec, adequate precision; Sqrt, square root.
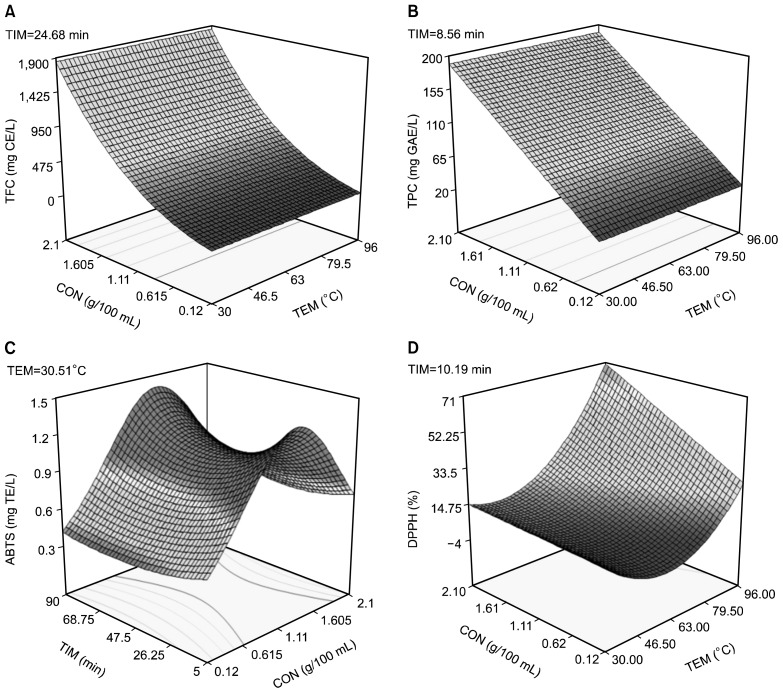
The response surface plot for the extraction of antioxidants is shown in Fig. 1. A rapid increase was observed in the concentration of the TFC (3,203%) and TPC (613%) of the extract as the powder concentration increased from 0.15 g/100 mL to 2.10 g/100 mL (Fig. 1A and 1B). An increase in the concentration of the ABTS radical scavenging activity of the extract was observed as the powder concentration increased from 0.12 g/100 mL to about 1.28 g/100 mL before a decline in the ABTS radical scavenging activity was observed (Fig. 1C). A possible reason for the reduction in the ABTS radical scavenging activity at very low solvent to powder ratio (high powder concentration) could be that increasing the solid mass leads to a decrease in the surface area available for the solvent to penetrate the substrate and solubilize the target molecules (11). The DPPH radical scavenging activity of the aqueous ginger extract was maximal at 96 °C, at all concentrations studied with the highest activity obtained at the highest ginger powder concentration of 2.1 g/100 mL (Fig. 1D). The maximum values for the antioxidant properties obtained from the response surface plots were 1,850.10 mg catechin equivalents/L (TFC), 190.33 mg gallic acid equivalent/L (TPC), and 1.26 Trolox equivalent/L (ABTS), 73.49% (peroxide scavenging activity), 65.30% (iron chelating activity), and 70.01% (DPPH).
Fig. 1.
Response surface graphs showing effect of extraction variables on antioxidant properties [(A) total flavonoid content (TFC), (B) total phenol content (TPC), (C) 2,2′-azinobis-(3-ethylbenzothiazoline-6-sulfonic acid) (ABTS) radical scavenging activity, and (D) 2,2-diphenyl-1-picrylhydrazyl (DPPH) radical scavenging activity] during aqueous extraction of ginger powder. TEM, temperature; CON, concentration; TIM, time; CE, catechin equivalent; GAE, gallic acid equivalent; TE, Trolox equivalent.
The single response optimization results for the aqueous extraction of antioxidants from ginger powder are shown in Table 2. The ABTS radical scavenging activity of the aqueous ginger extract was maximized at low temperature extraction. A high temperature extraction is required to maximize the DPPH radical scavenging activity of aqueous ginger extracts. Gunathilake and Rupasinghe (12) extracted fresh ginger rhizomes using hot water. They reported optimum extraction condition for the ginger polyphenols at a temperature above 60°C and a time greater than 60 min. However in their studies they did not look into the effect of ginger concentration. In this study, we observed that concentration was the only significant (P<0.05) factor for the aqueous extraction of ginger polyphenols and the effect was linear while the effects of temperature and time were not significant within the experimental range studied.
Table 2.
Single response optimisation conditions for aqueous ginger extraction
| Source | TFC | TPC | ABTS | Peroxide scavenging activity | Iron chelating activity | DPPH |
|---|---|---|---|---|---|---|
| TEM (°C) | 36.66ns | 37.31ns | 30.51 | –1) | – | 96 |
| CON (g/100 mL) | 2.10 | 2.10 | 1.28 | – | – | 2.1 |
| TIM (min) | 24.68ns | 8.56ns | 6.91 | – | – | 10.19ns |
TFC, total flavonoid content; TPC, total phenol content; ABTS, 2,2′-azinobis-(3-ethylbenzothiazoline-6-sulfonic acid) radical scavenging activity; DPPH, 2,2-diphenyl-1-picrylhydrazyl radical scavenging activity.
TEM, temperature; CON, concentration; TIM, time.
Not significant.
No prediction due to insignificant models.
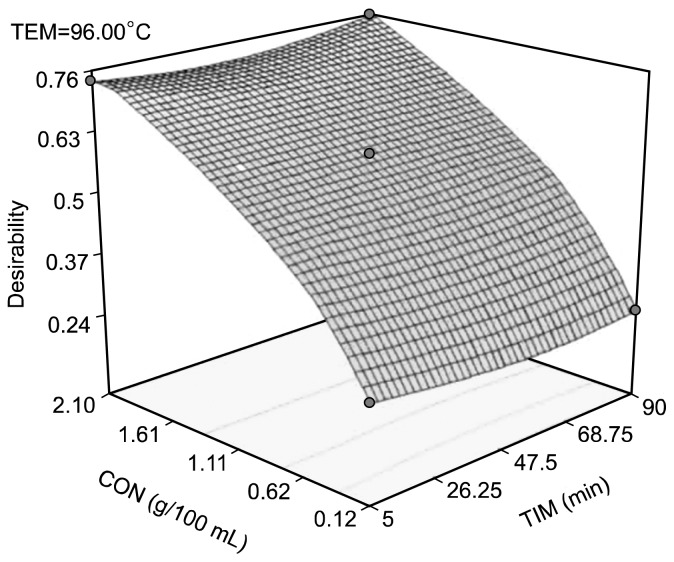
The multiresponse optimization condition for aqueous extraction of ginger powder antioxidant based on the experimental range studied was 96°C, 2.10 g/100 mL, and 90 min (Fig. 2). The multiresponse optimization was done using the single response models obtained for TPC, TFC, ABTS radical scavenging activity, and DPPH radical scavenging activity. The confirmation results for the multiresponse optimization were within the expected prediction interval values except for the TPC. The value of TPC was higher than the expected 95% confidence interval high (Table 3). However, since the goal of the multiresponse optimization is to maximize these antioxidant property this value is acceptable (5).
Fig. 2.
Response surface graph showing multi-response optimisation conditions for aqueous extraction of antioxidant from ginger powder. TEM, temperature; CON, concentration; TIM, time.
Table 3.
Confirmation run for aqueous ginger extraction (n=3)
| Response | Prediction | 95% CI low | 95% CI high | Validation |
|---|---|---|---|---|
| DPPH (%) | 70.01 | 49.34 | 90.68 | 64.47±1.40 |
| TPC (mg GAE/L) | 190.33 | 151.60 | 229.05 | 358.45±37.10 |
| TFC (mg CE/L) | 1,850.09 | 1,133.84 | 2,809.67 | 2,216.67±520.42 |
| ABTS (mg TE/L) | 0.90 | 0.67 | 1.27 | 0.91±0.016 |
| Peroxide scavenging activity (%) | –1) | – | – | 86.32±2.00 |
| Iron chelating activity (%) | – | – | – | 62.79±0.034 |
CI, confidence interval; DPPH, 2,2-diphenyl-1-picrylhydrazyl radical scavenging activity; TPC, total phenol content; TFC, total flavonoid content; ABTS, 2,2′-azinobis-(3-ethylbenzothiazoline-6-sulfonic acid) radical scavenging activity; GAE, gallic acid equivalent; CE, catechin equivalent; TE, Trolox equivalent.
No prediction due to insignificant models.
The regression parameters for prediction of antioxidant from aqueous ginger extract are shown in Table 4. The table shows the R2, Q2, and root mean square error for the predictions. The OLSR, PCR, and PLSR gave a R2 of 0.926, 0.818, and 0.713, respectively and a Q2 of 0.497, 0.284, and 0.702 for TFC prediction, respectively. A R2 of 0.866, 0.763, and 0.753, respectively and Q2 of 0.526, 0.524, and 0.748, respectively were obtained for OLSR, PCR, and PLSR for the prediction of TPC. The A610 property of the aqueous ginger extract gave a good indication of the TFC of the extract with an R2 and Q2 of 0.713 and 0.702, respectively. The A610 property of the extract also gave a good indication of the TPC of the extract with an R2 and Q2 of 0.753 and 0.748, respectively (Table 4). Makanjuola et al. (5) had reported that the A610 or A510 properties of an aqueous tea-ginger extract could give an indication of its TFC and TPC. The PCR and PLSR model provided a good indication of the DPPH property of the extract with an R2 of 0.905 and 0.818, respectively. The PLSR model using the A510, L* property of the extract was able to give a moderate explanation of the ABTS radical scavenging activity of the aqueous ginger extract (R2=0.521, Q2=0.417). Amongst the three multivariate regression used, the PLSR gave the simplest model with better predictive quality.
Table 4.
Regression parameters for antioxidant prediction in aqueous ginger extract
| Components1) | R2 | Q2 | RMSE | |
|---|---|---|---|---|
| TFC | ||||
| OLSR | L*, a*, b*, hue, chroma, pH, redox potential, A510, A610, A510/610, hue index | 0.926 | 0.497 | 354.857 |
| PCR | L*, b*, hue, chroma, pH, A610, hue index | 0.818 | 0.284 | 456.251 |
| PLSR | A610 | 0.713 | 0.702 | 443.533 |
| TPC | ||||
| OLSR | L*, a*, b*, hue, chroma, pH, redox potential, A510, A610, A510/610, hue index | 0.866 | 0.526 | 42.516 |
| PCR | L*, b*, A610, hue index | 0.763 | 0.524 | 41.260 |
| PLSR | A610 | 0.753 | 0.748 | 36.467 |
| DPPH | ||||
| OLSR | L*, a*, b*, hue, chroma, pH, redox potential, A510, A610, A510/610, hue index | 0.967 | 0.324 | 6.744 |
| PCR | L*, b*, hue, chroma, pH, A610, hue index | 0.905 | 0.644 | 9.305 |
| PLSR | b*, chroma, A610 | 0.818 | 0.672 | 9.993 |
| ABTS | ||||
| OLSR | L*, a*, b*, hue, chroma, pH, redox potential, A510, A610, A510/610, hue index | 0.688 | 0.321 | 0.183 |
| PCR | L*, b*, A610, hue index | 0.595 | 0.260 | 0.152 |
| PLSR | L*, A510 | 0.521 | 0.417 | 0.143 |
| 1/(Peroxide scavenging activity)2 | ||||
| OLSR | L*, a*, b*, hue, chroma, pH, redox potential, A510, A610, A510/610, hue index | 0.445 | −0.175 | 0.000849 |
| PCR | L*, b*, A610, hue index | 0.103 | −0.184 | 0.000788 |
| PLSR | –2) | – | – | – |
| 1/(Iron chelating activity)2 | ||||
| OLSR | L*, a*, b*, hue, chroma, pH, redox potential, A510, A610, A510/610, hue index | 0.843 | −0.267 | 0.000135 |
| PCR | L*, b*, pH, A610, hue index | 0.451 | −0.219 | 0.000191 |
| PLSR | – | – | – | – |
TFC, total flavonoid content; TPC, total phenol content; DPPH, 2,2-diphenyl-1-picrylhydrazyl radical scavenging activity; ABTS, 2,2′-azinobis-(3-ethylbenzothiazoline-6-sulfonic acid) radical scavenging activity; RMSE, root mean square error; OLSR, ordinary least square regression; PCR, principal component regression; PLSR, partial least square regression.
The component column shows the predictors present in the different regression equations.
No suitable model was found because the antioxidant property had no positive Q2 with any of the PLSR components.
This study showed that TEM, CON, and TIM influenced the extraction of antioxidants from ginger powder; however the powder concentration had the highest influence amongst the variable studied. Although an increase in ginger concentration from 0.12 to 2.10 g/100 mL led to an increase in the phytochemical content of the ginger extract (TPC and TFC), this does not apply to ABTS radical scavenging activity of the ginger extract as this activity was maximised at a concentration of 1.28 g/100 mL, after which a decline was observed. This result suggests that at a ‘particular concentration’, increasing the concentration of phytochemicals further may not necessarily result in increased antioxidant activity. The multiresponse optimization condition for aqueous extraction of ginger powder antioxidant based on the experimental range studied was 96°C, 2.10 g/100 mL, and 90 min. The colour property could also be exploited in rapid estimation of some antioxidant properties of the aqueous ginger extract.
Footnotes
AUTHOR DISCLOSURE STATEMENT
The authors declare no conflict of interest.
REFERENCES
- 1.Li Y, Hong Y, Han Y, Wang Y, Xia L. Chemical characterization and antioxidant activities comparison in fresh, dried, stir-frying and carbonized ginger. J Chromatogr B Analyt Technol Biomed Life Sci. 2016;1011:223–232. doi: 10.1016/j.jchromb.2016.01.009. [DOI] [PubMed] [Google Scholar]
- 2.Makanjuola SA, Enujiugha VN, Omoba OS, Sanni DM. Combination of antioxidants from different sources could offer synergistic benefits: a case study of tea and ginger blend. Nat Prod Commun. 2015;10:1829–1832. [PubMed] [Google Scholar]
- 3.Singletary K. Ginger: an overview of health benefits. Nutrition Today. 2010;45:171–183. doi: 10.1097/NT.0b013e3181ed3543. [DOI] [Google Scholar]
- 4.Semwal RB, Semwal DK, Combrinck S, Viljoen AM. Gingerols and shogaols: important nutraceutical principles from ginger. Phytochemistry. 2015;117:554–568. doi: 10.1016/j.phytochem.2015.07.012. [DOI] [PubMed] [Google Scholar]
- 5.Makanjuola SA, Enujiugha VN, Omoba OS, Sanni DM. Optimization and prediction of antioxidant properties of a tea-ginger extract. Food Sci Nutr. 2015;3:443–452. doi: 10.1002/fsn3.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gunathilake KDPP, Rupasinghe HPV. Inhibition of human low-density lipoprotein oxidation in vitro by ginger extracts. J Med Food. 2014;17:424–431. doi: 10.1089/jmf.2013.0035. [DOI] [PubMed] [Google Scholar]
- 7.Moure A, Cruz JM, Franco D, Domínguez JM, Sineiro J, Domínguez H, Núñez MJ, Parajó JC. Natural antioxidants from residual sources. Food Chem. 2001;72:145–171. doi: 10.1016/S0308-8146(00)00223-5. [DOI] [Google Scholar]
- 8.Kamuf W, Nixon A, Parker O, Barnum GC., Jr Overview of caramel colors. Cereal Foods World. 2003;48:64–69. [Google Scholar]
- 9.Goodner KL, Wampler B. [accessed Oct 2014];Measuring tea color using a simple spectrometric assay. 2008 http://www.synergytaste.com/sites/synergytaste.com/files/SEN-TN-0002-Measuring_Tea_Color_Using_A_Simple_Spectrometric_Assay.pdf.
- 10.McGuire RG. Reporting of objective color measurements. HortScience. 1992;27:1254–1255. [Google Scholar]
- 11.Destandau E, Michel T, Elfakir C. Microwave-assisted extraction. In: Rostagno MA, Prado JM, editors. Natural Product Extraction: Principles and Applications. RSC Publishing; Cambridge, UK: 2013. pp. 113–156. [DOI] [Google Scholar]
- 12.Gunathilake KDPP, Rupasinghe HPV. Optimization of water based-extraction methods for the preparation of bioactive-rich ginger extract using response surface methodology. Eur J Med Plants. 2014;4:893–906. doi: 10.9734/EJMP/2014/10322. [DOI] [Google Scholar]