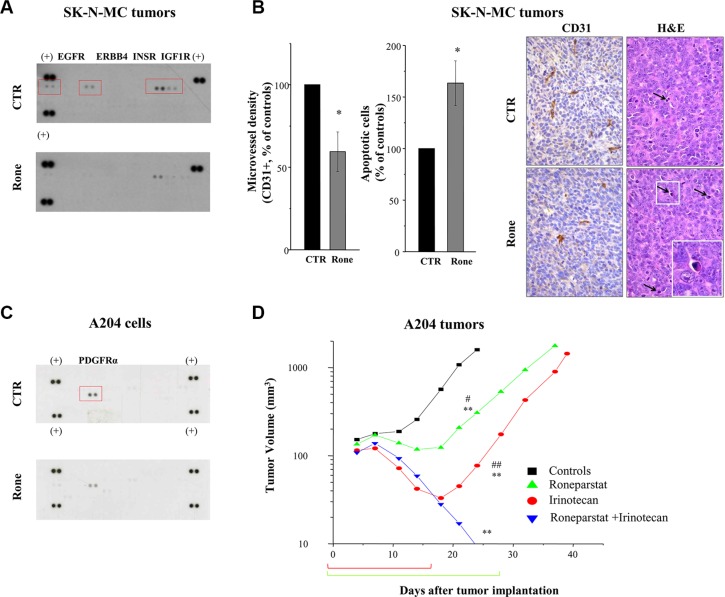
Figure 6. In vivo activity of roneparstat, alone and in combination, against sarcoma xenografts in mice.
(A), (B) Pharmacodynamic effect on RTKs in SK-N-MC xenografts associated with angiogenesis inhibition and apoptosis induction. Tumor xenografts-bearing mice were administered with vehicle (CTR) or roneparstat at 60 mg/kg (2qdx5/w)(Rone). After 16 days, tumors were removed and processed for proteomic profiling with phospho-RTK array (A) or immunohistochemical and histological analysis (B). For immunohistochemical detection of microvessel density, formalin fixed and paraffin embedded tumor sections were probed with an antibody recognizing CD31-positive cells. In parallel, tumor sections were stained with Hematoxylin-Eosin (H & E) for morphological detection of apoptotic cells. Columns, mean percentage of controls ± SE. On the right, representative images of CD31 and H&E staining. Arrows indicate apoptotic cells with typical morphological features: shrinkage and fragmentation into membrane-bound apoptotic bodies. Original magnification 400X; insert, 1000X. (C) Reduced constitutive phosphorylation of PDGFRα in roneparstat-treated A204 rhabdoid cells. After 48 h of incubation in the presence of solvent or 1 mg/ml roneparstat in serum-free medium, cells were lysed and processed for proteomic profiling with phospho-RTK array. (+), reference spots. (D) Enhancement of antitumor efficacy against A204 rhabdoid xenografts by combined treatment with irinotecan. Irinotecan (50 mg/kg) was administered i.v. with an intermittent treatment schedule q4dx4; roneparstat (60 mg/kg) was administered s.c., 2qdx6/w, for 4 weeks. Brackets under abscissa indicate the treatments' timeframe. *P < 0.05, **P < 0.01, drug-treated versus control tumors, #P < 0.05, ##P < 0.01 versus drug combination at day 24 after tumor implantation.