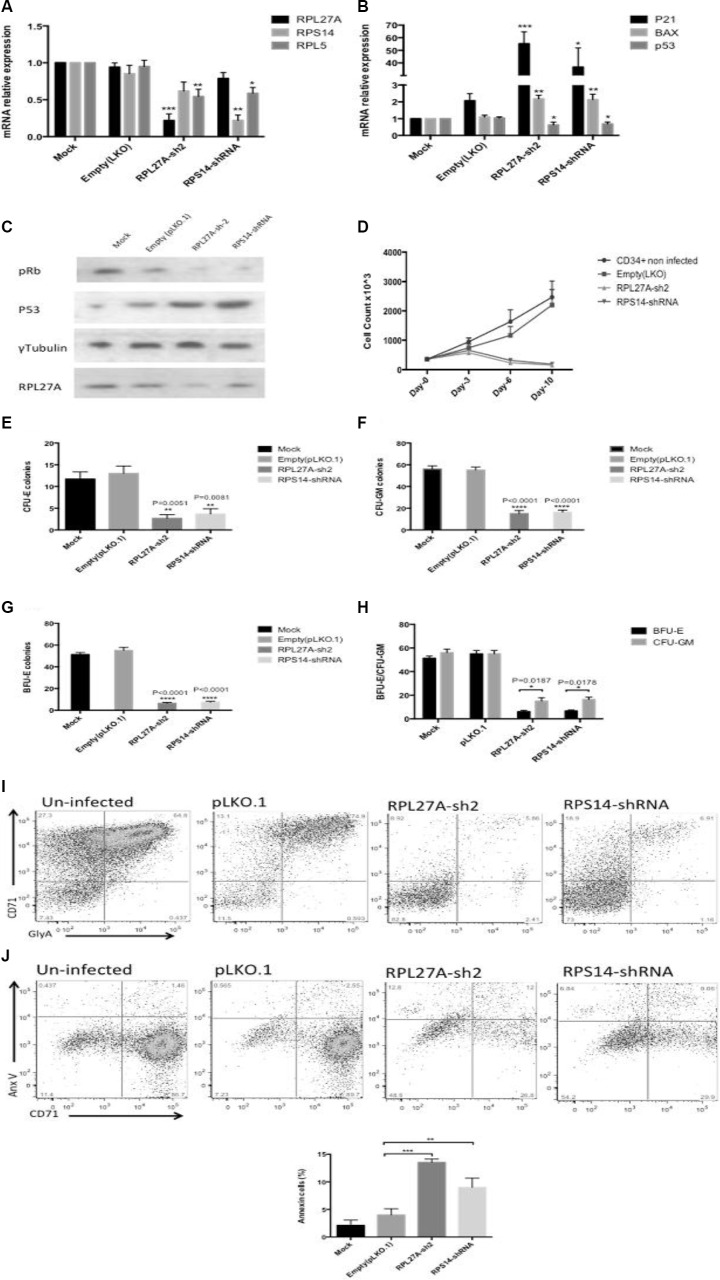
Figure 7. RPL27A knockdown in normal CD34+ cells blocks haematopoietic stem cell proliferation and differentiation.
Normal CD34+ cells underwent infection either with control, RPL27A-shRNA or RPS14-shRNA and samples collected for analysis on Day 6. A significant reduction in RPL27A and RPL5 mRNA expression was evident following RPL27A-shRNA infection. Reduced RPS14 and RPL5 expression followed RPS14 shRNA infection (A). Upregulated expression of p53 mRNA and both p21 and Bax was induced following either RPL27A or RPS14 shRNA infection (B). Depletion of RPL27A and RPS14 also resulted in increased p53 protein expression and hypophosphorylation of phospho-Retinoblastoma (pRB) protein (C). Enumeration of cells at 48-hour intervals post infection demonstrated reductions in cell numbers following infection with either RPL27A-sh2 or RPS14-shRNA compared to controls (D). Methylcellulose colony formation assays were used to assess frequencies of progenitor cells following infection with either control, RPL27A-shRNA or RPS14 shRNA. In both RPL27A and RPS14 deficient cells, the most prominent reduction was in the erythroid lineage. Data is representative of 4 independent experiments, bars represent the mean ± SEM. CFU: colony forming unit including all colony types except erythroid; CFU-E: colony forming unit erythroid only (E–H). Flow cytometry was performed on cells harvested on Day 10/11 post infection. Cells were labelled with a combination of conjugated fluorometric antibodies including live/dead staining (780), an immature erythroid marker (CD71) and mature erythroid marker (Glycophorin A (GlyA)). Following either RPL27A-shRNA or RPS14 shRNA infection, a significant reduction in erythroid cells was evident compared with controls (I + J).