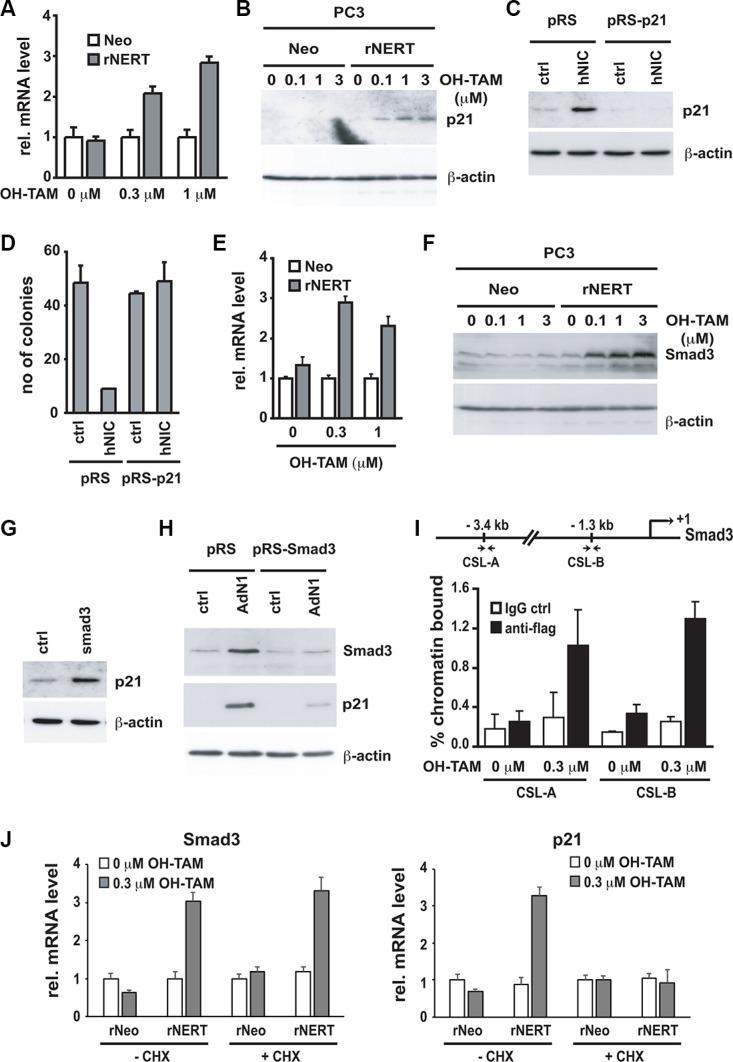
Figure 4. p21WAF1/Cip1 mediates Notch1-induced growth arrest and is controlled by Notch1 through a Smad3 dependent mechanism.
(A and B) PC3 cells stably infected with a retroviral vector expressing the flag tagged-activated Notch1 protein fused to the human estrogen receptor (rNERT), or control virus (Neo) were treated for 3 days with 4-hydroxytamoxifen at the indicated concentrations. Expression of p21WAF1/Cip1 was analyzed by real time RT-PCR (using 36β4 mRNA for normalization) (A) and by immuno-blotting (B). (C) PC3 cells were stably infected with a retrovirus expressing an shRNA against p21WAF1/Cip1 (pRS-p21 n°1) or empty vector control (pRS). Cells were subsequently transfected with an expression vector for activated Notch1 (hNIC) together with a neomycin resistance gene, or empty vector control (ctrl). Twenty four hours later, cells were analyzed by immuno-blotting for p21WAF1/Cip1 expression, with β-actin for normalization. (D) PC3 cells plus/minus p21WAF1/Cip1 knock-down and activated Notch1 expression as in the previous panel were replated (at 8 hours after transfection) at low density in triplicate dishes (1000 cells per 6 cm dish) in the presence of neomycin for selection of transfected cells. Numbers of colonies per dish were counted 10 days later. (E and F) The same PC3 cells with inducible Notch1 activity as in (A and B) were analyzed for Smad3 expression by real time RT-PCR (using 36β4 mRNA for normalization) (E) and immunoblotting (F). (G) PC3 cells were transiently transfected with an expression vector for Smad3 (Smad3) or empty vector control (ctrl) for 48 hours, followed by immunoblot analysis of p21WAF1/Cip1 and β-actin proteins. (H) PC3 cells were stably infected with a retrovirus expressing shRNA against Smad3 (pRS-Smad3 n°1) or empty vector control (pRS) and subsequently infected with an activated Notch1 expressing adenovirus (AdN1) or GFP-expressing control (ctrl). Twenty four hours later, cells were analyzed by immunoblotting for levels of Smad3 and p21WAF1/Cip1 proteins with β-actin for normalization. (I) The same PC3 cells with inducible Notch1 activity as in (A and B) were processed for chromatin immunoprecipitation with antibodies specific for flag or non-immune IgG control followed by PCR amplification (50 cycles) of various regions of the Smad3 promoter as indicated in the schematic above. The sequences of the two predicted RBP-binding sites (CSL-A and CSL-B) are, respectively, 5′-CTAATGGGAAAATAA-3′ and 5′-GGGGTGGGAGATTCC-3′. Un-precipitated chromatin preparations were similarly analysed and used as “input DNA” control. Binding of the rNERT-flag fusion protein to its two predicted CSL binding sites in the Smad3 promoter was quantified by chromatin immuno-precipitation assay and real time PCR. The amount of precipitated DNA was calculated relative to the total input chromatin, and expressed as percentage of the total according to the following formula (Frank et al. 2001) : % total = 2ΔCtx5 where ΔCt = Ct (input)-Ct (immuno-precipitation). Ct: cycle threshold. (J) PC3 cells expressing the rNERT fusion protein were treated, in parallel with control cells (Neo), for 24 hours with 4-hydroxytamoxifen in presence or absence of cycloheximide (CHX, 10 mg/ml, added 2 hours before 4-hydroxytamoxifen treatment). Expression of Smad3 (left panel) and p21WAF1/Cip1 (right panel) was analyzed by real time RT-PCR (using 36β4 mRNA for normalization).