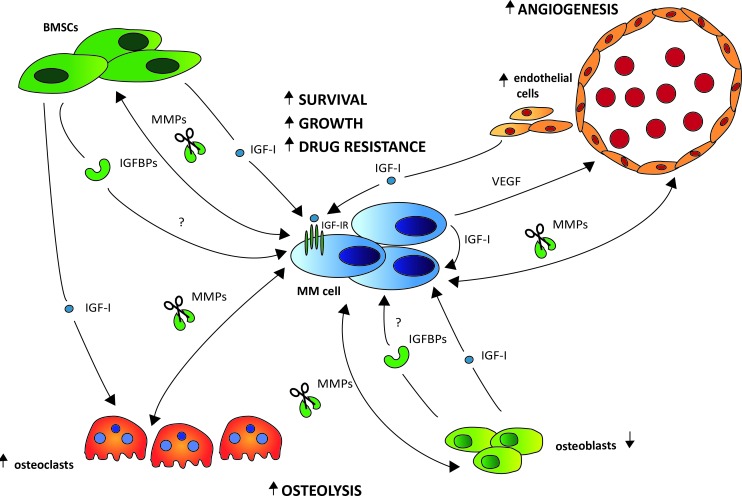
Figure 3. The role of the IGF system in the bi-directional interactions of the MM cells and the different stromal cell types.
Upon interaction with the MM cells through adhesion molecules, IGF-I is abundantly produced by different BM stromal cell types, including the BM fibroblasts (BMSCs), osteoclasts (OCs), osteoblasts (OBs) and endothelial cells (ECs). Binding of IGF-I to the IGF-IR expressed on the surface of the MM cells will then foster MM cell survival, growth and drug resistance. The increased IGF-I secretion will also stimulate the MM cells to produce VEGF, leading to enhanced BM angiogenesis further fostering MM cell survival and growth. Moreover, IGF-I will also promote bone destruction by enhancing OC migration and activation. This bone destruction will in turn result in the release of numerous growth and survival factors embedded in the bone matrix, thus further driving the vicious cycle. In the extracellular environment, the majority of IGF is known to be bound to one of six IGF-binding proteins (IGFBP1-6), leaving only a minor fraction of total IGF free and accessible for receptor activation. Although preliminary evidence indicates an abnormal IGFBP profile in MGUS and MM leading to an increased IGF-I bioavailability, the exact role of the IGFBPs in the pathobiology of MM and the major source in the BM niche remains to be investigated. Cleavage of the IGFBPs by protease, including matrix metalloproteinases (MMPs), also results in increased IGF-I bioavailability. In MM, interaction of the MM cells with the different stromal cell types strongly enhances the production of MMPs not only by the MM cells but also by the stromal cells.