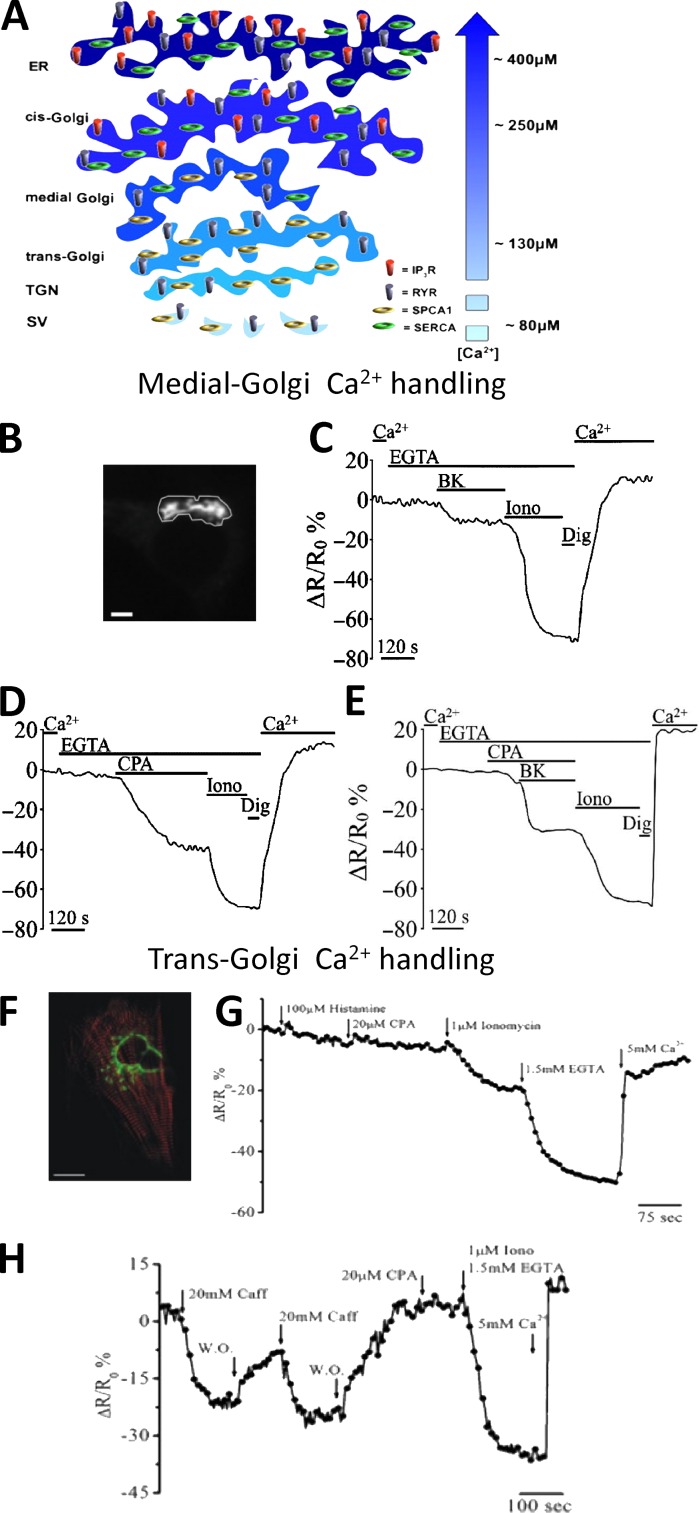
Figure 6.
The heterogeneity of GA in Ca2+ handling. (A) [Ca2+] and molecular toolkit along the secretory pathway. The GA can be divided in three distinct subcompartments: the cis-Golgi, with a luminal [Ca2+] around 250 µM, the medial Golgi, with a luminal [Ca2+] lower compared with that of the cis-Golgi (i.e., ∼150–200 µM), and trans-Golgi, with a luminal [Ca2+] around 130 µM. The efflux and influx Ca2+ toolkit is also shown. TGN, trans-Golgi network; SV, secretory vesicles (from Pizzo et al. [2011] with permission from Elsevier). (B–E) Ca2+ handling by medial Golgi in intact cells monitored with a targeted Cameleon probe: (B) the fluorescence microscope image of a medialGo-D1cpv–expressing SH-SY5Y cell. Bar, 10 µm. (C–E) SHSY-5Y cells were incubated in medium supplemented or not with 1 mM CaCl2 or 300 µM EGTA and challenged with the indicated stimuli: (C) bradykinin (BK), demonstrating the presence of an IP3 sensitive pool; (D) cyclopiazonic acid (CPA), demonstrating the presence of the SERCA pump; (E) ionomycin (Iono), a ionophore demonstrating the presence of another molecular component besides IP3Rs and SERCA, such as SPCA1 (from Wong et al. [2013] with permission from Oxford University Press). (F–H) Ca2+ handling by trans-Golgi in single intact cells monitored with a targeted Cameleon probe: (F) confocal microscopy image of a cardiomyocyte cell expressing transGo-D1cpv and the mRFP-Zasp construct (red). Bar, 10 µm. (G and H) HeLa cells (G) and cardiac myocytes (H) were exposed to different stimuli demonstrating that this compartment is enriched of SPCA1 (ionomycin-sensitive pool) and RyRs (caffeine-sensitive pool), but neither SERCA (CPA-sensitive pool) nor IP3Rs (histamine-sensitive pool) are present (from Lissandron et al. [2010] with permission from the National Academy of Sciences).