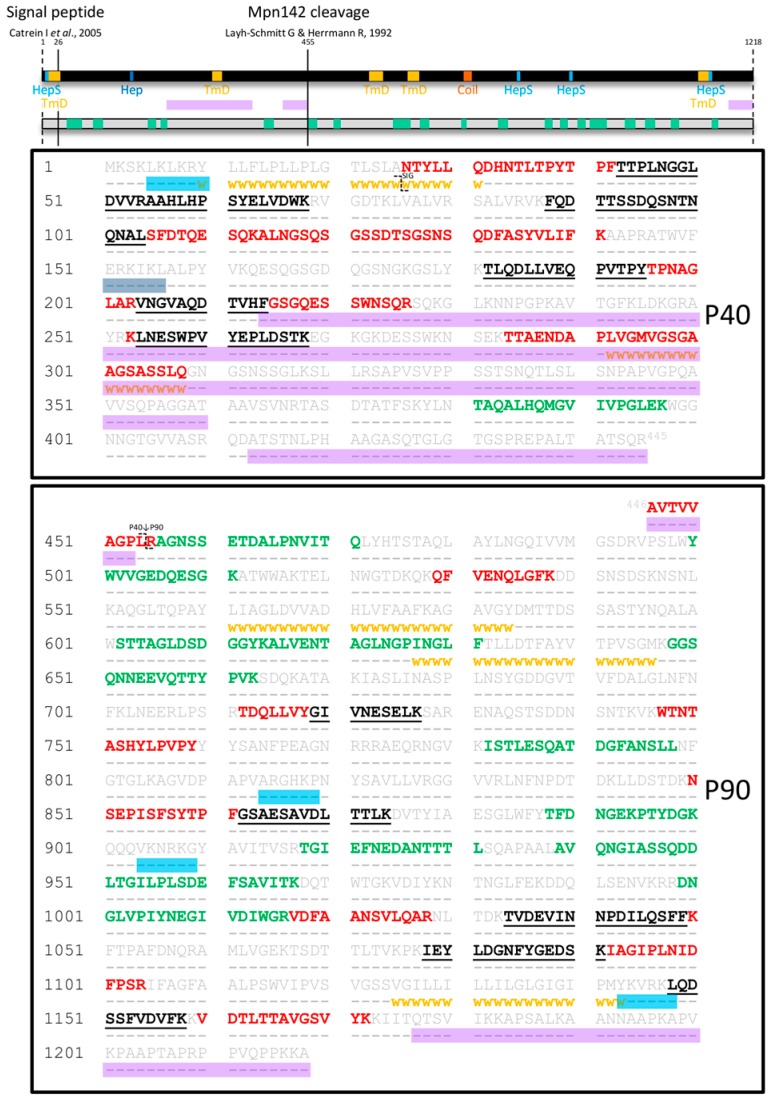
Figure 1.
Peptides identified in surface proteome analysis of Mpn142. Full length Mpn142 is represented as a black bar and its amino acid sequence (grey) is shown underneath. Tryptic peptides identified by shaving the surface of M. pneumoniae are coloured green while tryptic peptides derived from a 2D gel loaded with biotinylated (surface) proteins of M. pneumoniae are coloured red. Tryptic peptides that were common to both surface analyses are coloured black and underlined. Bioinformatics tools were used to predict coiled-coils (orange box; COILS), transmembrane domains (yellow squares/ⱳⱳⱳⱳⱳ; TMpred), disordered regions (purple boxes; PONDR®: VSL2 and VSL3 predictor), heparin-binding motifs (dark blue box; ScanProsite with “X-[HRK]-[HRK]-X-[HRK]-X” motif) and motifs implicated in heparan sulfate binding (light blue boxes; ScanProsite with “X-[HRK]-X-[HRK]-[HRK]-X” motif). The two previously reported cleavage sites after amino acid 25 and 454 are indicated as unbroken lines across the black bar and with the symbol ʅ in the sequence.