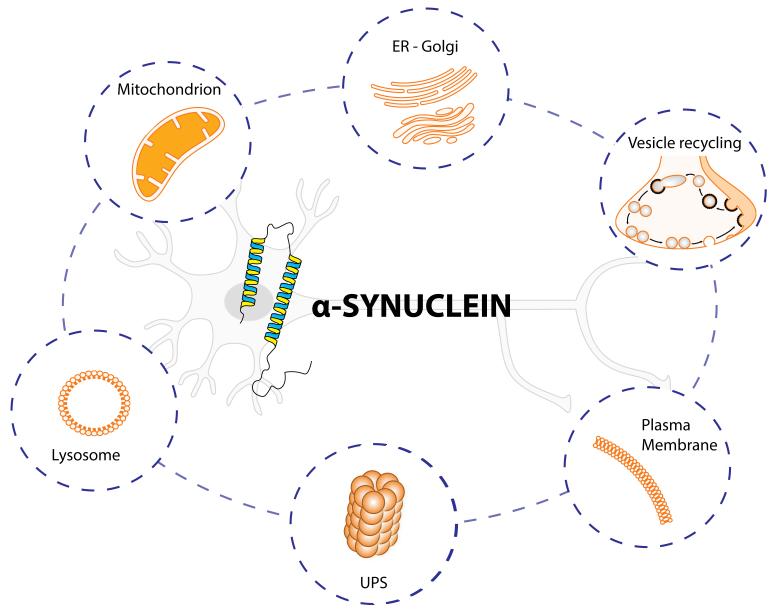
Figure 3. Schematic summary of established interactions between α-synuclein and cellular components.
The figure highlights six different intracellular pathways affected by α-synuclein (α-syn). The protein α-syn is enriched at the pre-synaptic terminals of almost all types of neurons in the brain, where it participates in the vesicle recycling, thereby modulating synaptic function. α-syn can be degraded by the ubiquitin-proteasome system (UPS) and inside the lysosomes. α-syn interacts strongly with membranes, such as plasma membrane and mitochondrion. If misfolded, α-syn forms distinct structures that are prone to aggregation, first into oligomers, then into larger structures. It is now believed that α-syn oligomers are the toxic form that may impair basic neuronal processes, such as ER-Golgi trafficking, lysosome and UPS functions, reduced mitochondrial activity and alter the plasma membrane through the pore/perforations that can dysregulate calcium and cation homeostasis.