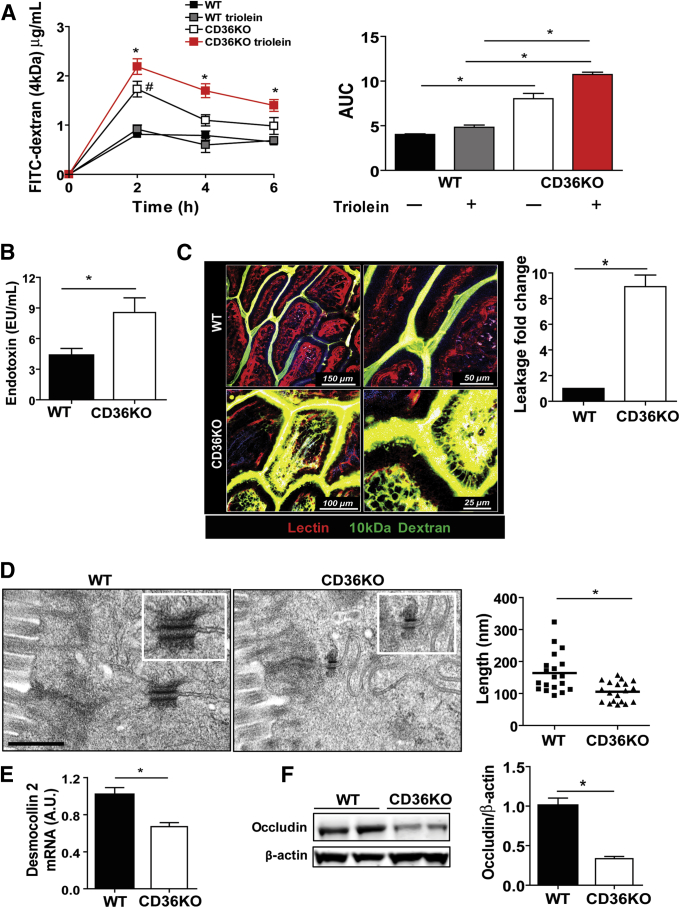
Figure 2.
Gut barrier permeability is impaired in CD36KO mice. (A) Plasma level of FITC-dextran (4 kDa) measured at indicated times after its intragastric administration. A week later, same mice group received triolein bolus (4.5 μL/g body weight) 30 minutes before FITC-dextran. #WT versus CD36KO mice, *WTtriolein versus CD36KOtriolein mice. Levels in WT mice did not change with or without triolein bolus. Intestinal permeability was increased in all CD36KO mice at 2 hours (P = .04; Ptriolein = .032) and remained increased at 4 hours (P = .043) and 6 hours (P = .041) in CD36KO mice given triolein. Right panel shows area under the curve (AUC) for CD36KO and CD36KOtriolein mice was increased compared with appropriate controls (P < .001 and P = .005, respectively) and before as compared with after triolein challenge (P < .001). (B) Measurement of endotoxin in plasma of WT and CD36KO mice 4 hours after triolein challenge (n = 4/genotype), P = .03. (C) Two-photon microscopy optical sections showing leakage across the epithelium; fluorescein-dextran (10 kDa) (green) was administered intraluminally to intestines of anesthetized mice (n = 3/genotype) and DyLight 594–conjugated tomato lectin (red), a vascular marker, by retro-orbital injection 10 minutes before imaging from the luminal surface. Dextran leakage is observed in CD36KO mice but not in WT mice (P < .01). Quantification of leakage is expressed as fold change of FITC-dextran fluorescence inside the villus versus fluorescence between epithelial cells measured in 5 random villi/mouse. Blue fluorescence, 2-harmonic generation; light purple fluorescence, autofluorescence. Scale bars: WT, 150 and 50 μm; CD36KO, 100 and 25 μm. (D) Electron microscopy images showing shortened desmosomes in intestinal epithelium of CD36KO mice. Scale bar: 500 nm. Graph shows quantification of decrease in desmosome length (n = 20/genotype, P = .001). (E) Expression of desmosomal protein desmocollin 2 is decreased in intestines of CD36KO mice compared with controls (P = .01, n = 8/genotype). (F) Level of tight junctional protein occludin measured in intestinal lysates by immunoblotting and densitometry quantification of change in occludin/β-actin as compared with WT control (P = .01) (n = 4/genotype). Data are representative of 3 (A–E) and 2 (F) experiments. Bar graphs show means ± SEM.