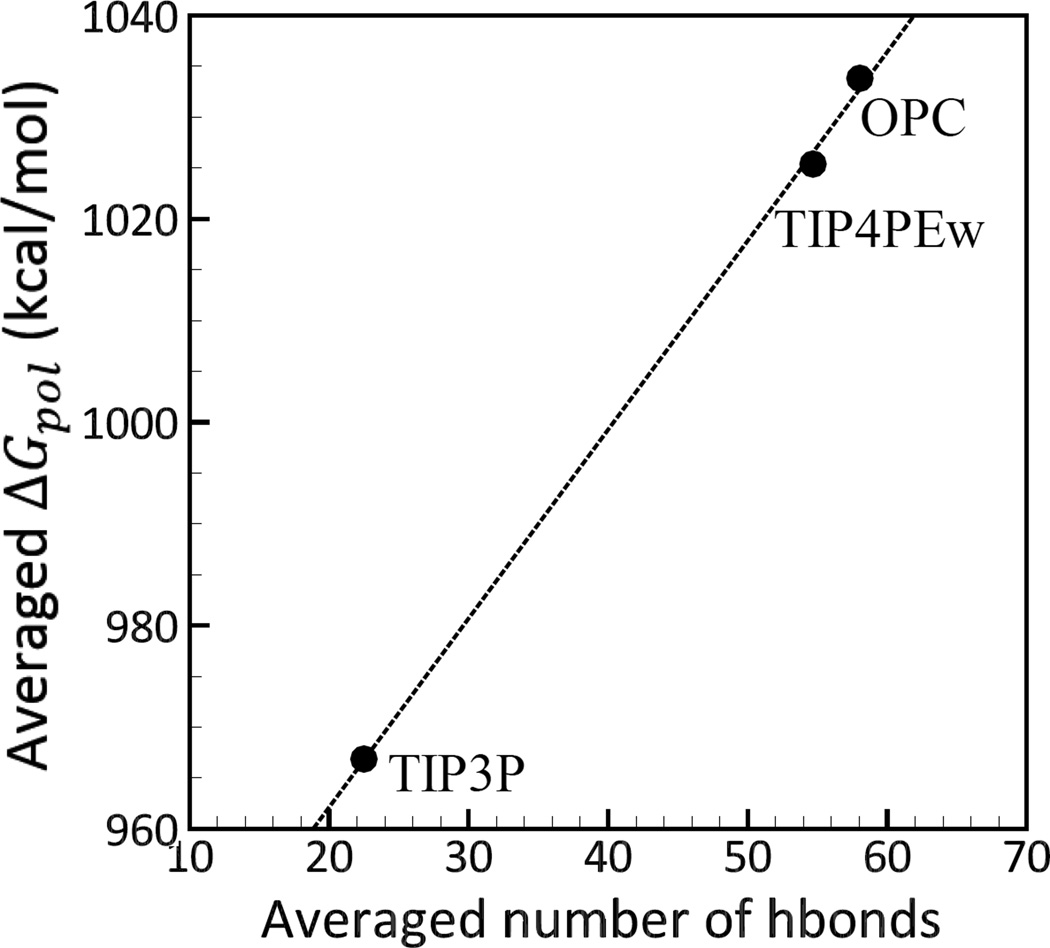
Figure 3.
Correlation between the electrostatic sol-vation free energies ΔGpol and the number of hydrogen bonds formed between the complexes and the explicit solvent models (TIP3P, TIP4PEw and OPC). The ΔGpol values shown for each model are averages over complexes, and the number of hydrogen bonds represents averages over MD trajectory and over complexes. The hydrogen bond is considered to be formed if the distance between the acceptor (A) and the donor (D) atoms is smaller than 3Å, and angle D-H-A is greater than 135°.114 Connecting lines are shown to guide the eye.