Fig. 1.
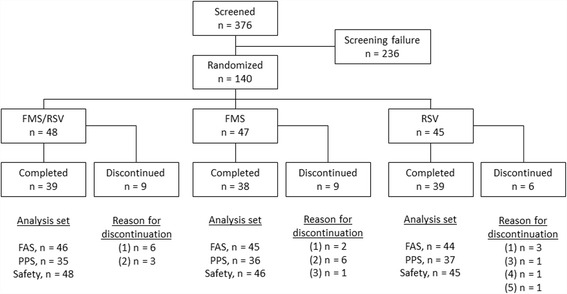
Subject disposition and reasons for study discontinuation. Reasons for discontinuation included (1) withdrawal of consent, (2) protocol violations, (3) lack of efficacy, (4) adverse events, and (5) other reasons. FMS: fimasartan; RSV, rosuvastatin. FMS/RSV: fimasartan 120 mg/rosuvastatin 20 mg treatment; FMS: fimasartan 120 mg alone treatment; RSV: rosuvastatin 20 mg alone treatment; FAS: full analysis set; PPS: per-protocol set
