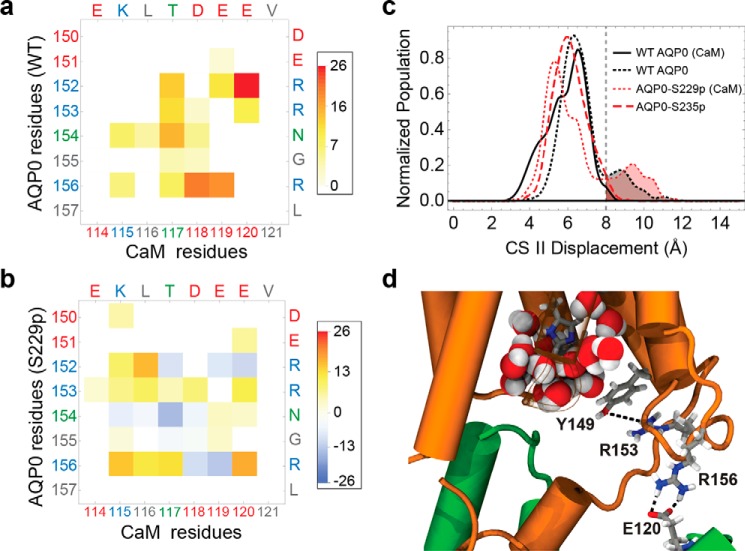
FIGURE 3.
Phosphorylation of AQP0 modified the permeability of the channels by modulating the CaM interaction. a, matrix representation of the interprotein contacts formed between WT AQP0 residues and CaM residues at the cytosolic arginine loop. The color scale indicates the average number of atomic-level contacts over a MD trajectory (see “Experimental Procedures”). Residues are color-labeled on the axis by type (red, negative; blue, positive; green, polar; gray, nonpolar). b, matrix plot of contact differences between CaM-bound AQP0-S229P and CaM-bound WT AQP0 (subtraction of the CaM-bound WT AQP0 from the CaM-bound AQP0 S229P matrix). Darker red and darker blue indicate, respectively, contact formation and contact elimination upon Ser-229 phosphorylation. In the color scale, positive values correspond to a net increase in the average number of contacts, and negative values correspond to a net decrease in the number of contacts upon Ser-229 phosphorylation. c, CSII distance distributions for different AQP0 variants, indicating the size of the pore opening to the cytosolic vestibule. The different AQP0 variants consist of WT and phosphorylated AQP0 with and without CaM. d, configuration snapshot from an MD simulation of CaM-bound AQP0-S229P showing Arg-153 interacting with the Tyr-149 hydroxyl group, which displaces Tyr-149 away from the water pore. This is caused by the AQP0 Arg-156 interaction with CaM shifting from Asp-118 to Glu-120. An increase in the density of the AQP0 pore lumen waters (shown in filled sphere representation colored by atom type) can be seen near the cytoplasmic opening in response to the Tyr-149 displacement.