Abstract
Transforming growth factor beta 1 (TGF-beta 1), a homodimeric polypeptide (Mr 25,000), derives from inflammatory cells and acts as a chemoattractant for monocytes and fibroblasts. We report here that TGF-beta 1 is also the most potent chemoattractant yet described for human peripheral blood neutrophils. Recombinant TGF-beta 1 elicited dose-dependent directed migration of neutrophils under agarose that was inhibited in the presence of a neutralizing antibody to TGF-beta 1. Maximal chemotaxis was evoked by TGF-beta 1 at femtomolar concentrations, whereas conventional chemoattractants act at nanomolar concentrations: on a molar basis, TGF-beta 1 was 150,000 times more potent than fMet-Leu-Phe. In contrast, TGF-beta 1 provoked neither exocytosis nor the production of superoxide by neutrophils. We further analyzed the mechanism by which TGF-beta 1 elicits chemotaxis (GTPase activity, [Ca2+], and actin polymerization). In contrast to the conventional chemoattractant fMet-Leu-Phe, TGF-beta neither activated classic heterotrimeric guanine nucleotide-binding proteins nor provoked global mobilization of intracellular Ca2+. Chemoattraction by both fMet-Leu-Phe and TGF-beta 1 was inhibited by cycloheximide and actinomycin D. Moreover, chemotaxis in response to TGF-beta 1 was associated with the polymerization of actin. The selectivity and potency of TGF-beta 1 as a chemoattractant suggest that it elicits directed cell migration by means of a pathway that depends not on classic intracellular signals but on protein synthesis.
Full text
PDF
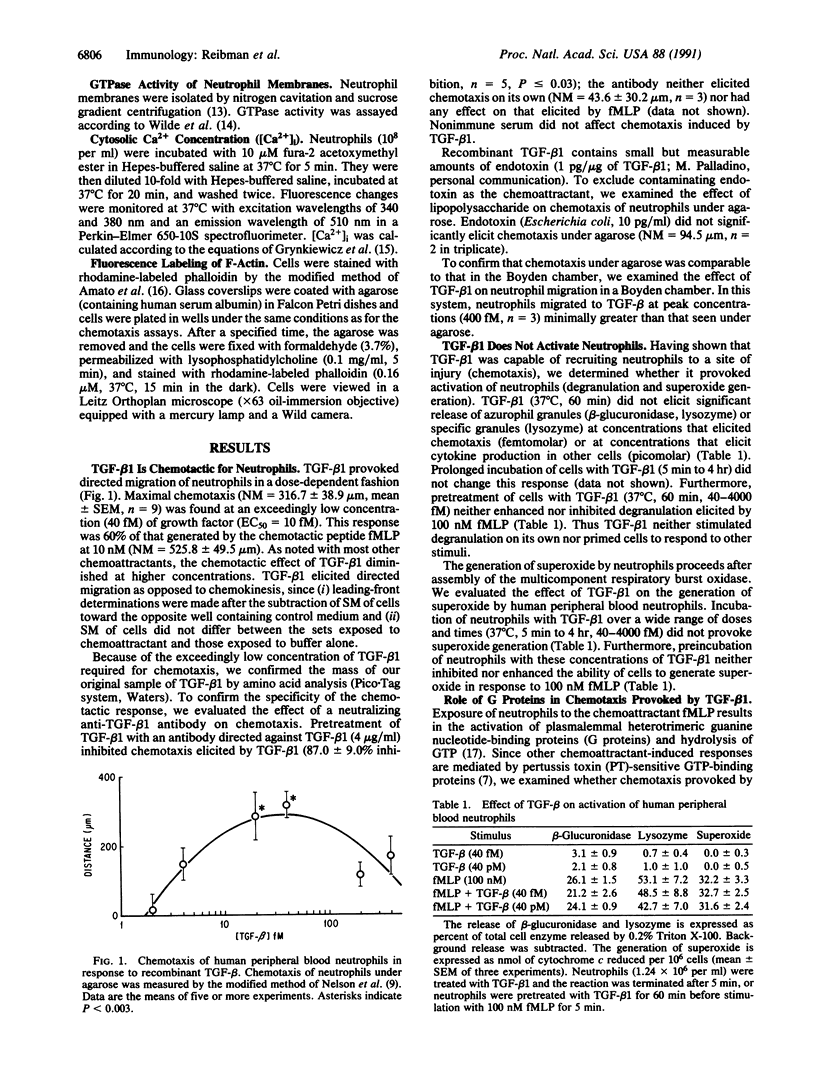
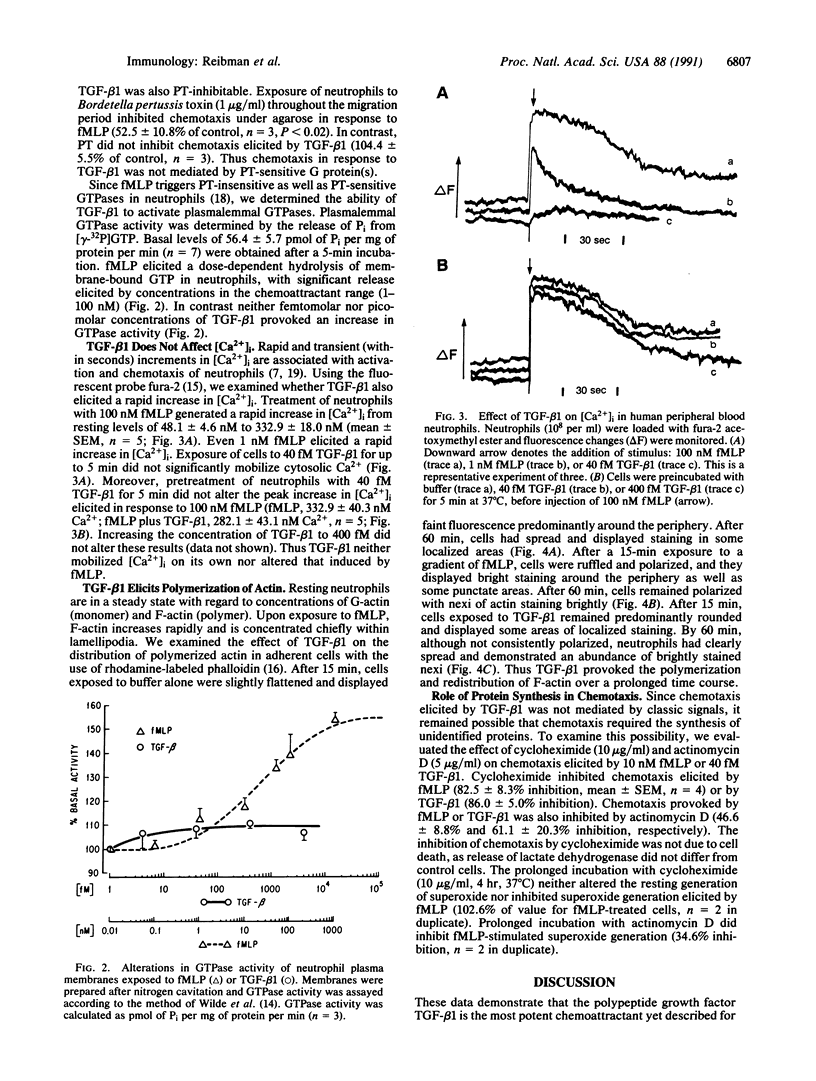
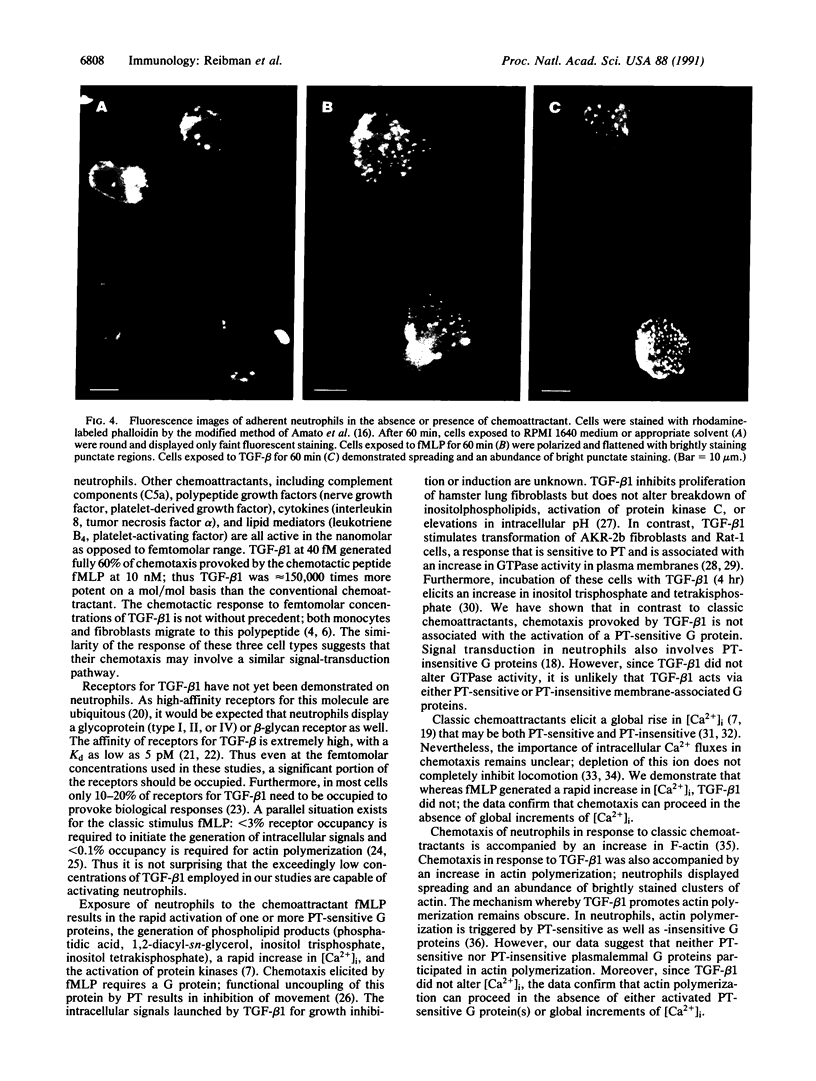

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amato P. A., Unanue E. R., Taylor D. L. Distribution of actin in spreading macrophages: a comparative study on living and fixed cells. J Cell Biol. 1983 Mar;96(3):750–761. doi: 10.1083/jcb.96.3.750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bengtsson T., Särndahl E., Stendahl O., Andersson T. Involvement of GTP-binding proteins in actin polymerization in human neutrophils. Proc Natl Acad Sci U S A. 1990 Apr;87(8):2921–2925. doi: 10.1073/pnas.87.8.2921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chambard J. C., Pouysségur J. TGF-beta inhibits growth factor-induced DNA synthesis in hamster fibroblasts without affecting the early mitogenic events. J Cell Physiol. 1988 Apr;135(1):101–107. doi: 10.1002/jcp.1041350114. [DOI] [PubMed] [Google Scholar]
- Cheifetz S., Bassols A., Stanley K., Ohta M., Greenberger J., Massagué J. Heterodimeric transforming growth factor beta. Biological properties and interaction with three types of cell surface receptors. J Biol Chem. 1988 Aug 5;263(22):10783–10789. [PubMed] [Google Scholar]
- Cheifetz S., Weatherbee J. A., Tsang M. L., Anderson J. K., Mole J. E., Lucas R., Massagué J. The transforming growth factor-beta system, a complex pattern of cross-reactive ligands and receptors. Cell. 1987 Feb 13;48(3):409–415. doi: 10.1016/0092-8674(87)90192-9. [DOI] [PubMed] [Google Scholar]
- Devreotes P. N., Zigmond S. H. Chemotaxis in eukaryotic cells: a focus on leukocytes and Dictyostelium. Annu Rev Cell Biol. 1988;4:649–686. doi: 10.1146/annurev.cb.04.110188.003245. [DOI] [PubMed] [Google Scholar]
- Gallin J. I., Wright D. G., Schiffmann E. Role of secretory events in modulating human neutrophil chemotaxis. J Clin Invest. 1978 Dec;62(6):1364–1374. doi: 10.1172/JCI109257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman D. W., Chang F. H., Gifford L. A., Goetzl E. J., Bourne H. R. Pertussis toxin inhibition of chemotactic factor-induced calcium mobilization and function in human polymorphonuclear leukocytes. J Exp Med. 1985 Jul 1;162(1):145–156. doi: 10.1084/jem.162.1.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein I. M., Horn J. K., Kaplan H. B., Weissmann G. Calcium-induced lysozyme secretion from human polymorphonuclear leukocytes. Biochem Biophys Res Commun. 1974 Sep 23;60(2):807–812. doi: 10.1016/0006-291x(74)90312-x. [DOI] [PubMed] [Google Scholar]
- Grotendorst G. R., Smale G., Pencev D. Production of transforming growth factor beta by human peripheral blood monocytes and neutrophils. J Cell Physiol. 1989 Aug;140(2):396–402. doi: 10.1002/jcp.1041400226. [DOI] [PubMed] [Google Scholar]
- Grynkiewicz G., Poenie M., Tsien R. Y. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem. 1985 Mar 25;260(6):3440–3450. [PubMed] [Google Scholar]
- Hong D., Stevens P. The role of protein synthesis in the chemotaxis and chemiluminescence of human polymorphonuclear leukocytes. Pharmacology. 1984;28(5):281–288. doi: 10.1159/000137975. [DOI] [PubMed] [Google Scholar]
- Howe P. H., Bascom C. C., Cunningham M. R., Leof E. B. Regulation of transforming growth factor beta 1 action by multiple transducing pathways: evidence for both G protein-dependent and -independent signaling. Cancer Res. 1989 Nov 1;49(21):6024–6031. [PubMed] [Google Scholar]
- Ignotz R. A., Massagué J. Transforming growth factor-beta stimulates the expression of fibronectin and collagen and their incorporation into the extracellular matrix. J Biol Chem. 1986 Mar 25;261(9):4337–4345. [PubMed] [Google Scholar]
- Korchak H. M., Vienne K., Rutherford L. E., Wilkenfeld C., Finkelstein M. C., Weissmann G. Stimulus response coupling in the human neutrophil. II. Temporal analysis of changes in cytosolic calcium and calcium efflux. J Biol Chem. 1984 Apr 10;259(7):4076–4082. [PubMed] [Google Scholar]
- Korchak H. M., Wilkenfeld C., Rich A. M., Radin A. R., Vienne K., Rutherford L. E. Stimulus response coupling in the human neutrophil. Differential requirements for receptor occupancy in neutrophil responses to a chemoattractant. J Biol Chem. 1984 Jun 25;259(12):7439–7445. [PubMed] [Google Scholar]
- Leof E. B., Proper J. A., Getz M. J., Moses H. L. Transforming growth factor type beta regulation of actin mRNA. J Cell Physiol. 1986 Apr;127(1):83–88. doi: 10.1002/jcp.1041270111. [DOI] [PubMed] [Google Scholar]
- Massagué J., Like B. Cellular receptors for type beta transforming growth factor. Ligand binding and affinity labeling in human and rodent cell lines. J Biol Chem. 1985 Mar 10;260(5):2636–2645. [PubMed] [Google Scholar]
- Meshulam T., Proto P., Diamond R. D., Melnick D. A. Calcium modulation and chemotactic response: divergent stimulation of neutrophil chemotaxis and cytosolic calcium response by the chemotactic peptide receptor. J Immunol. 1986 Sep 15;137(6):1954–1960. [PubMed] [Google Scholar]
- Molski T. F., Naccache P. H., Marsh M. L., Kermode J., Becker E. L., Sha'afi R. I. Pertussis toxin inhibits the rise in the intracellular concentration of free calcium that is induced by chemotactic factors in rabbit neutrophils: possible role of the "G proteins" in calcium mobilization. Biochem Biophys Res Commun. 1984 Oct 30;124(2):644–650. doi: 10.1016/0006-291x(84)91603-6. [DOI] [PubMed] [Google Scholar]
- Muldoon L. L., Rodland K. D., Magun B. E. Transforming growth factor beta modulates epidermal growth factor-induced phosphoinositide metabolism and intracellular calcium levels. J Biol Chem. 1988 Apr 15;263(11):5030–5033. [PubMed] [Google Scholar]
- Murthy U. S., Anzano M. A., Stadel J. M., Greig R. Coupling of TGF-beta-induced mitogenesis to G-protein activation in AKR-2B cells. Biochem Biophys Res Commun. 1988 May 16;152(3):1228–1235. doi: 10.1016/s0006-291x(88)80416-9. [DOI] [PubMed] [Google Scholar]
- Nelson R. D., Quie P. G., Simmons R. L. Chemotaxis under agarose: a new and simple method for measuring chemotaxis and spontaneous migration of human polymorphonuclear leukocytes and monocytes. J Immunol. 1975 Dec;115(6):1650–1656. [PubMed] [Google Scholar]
- Ohta H., Okajima F., Ui M. Inhibition by islet-activating protein of a chemotactic peptide-induced early breakdown of inositol phospholipids and Ca2+ mobilization in guinea pig neutrophils. J Biol Chem. 1985 Dec 15;260(29):15771–15780. [PubMed] [Google Scholar]
- Pelz C., Matsumoto T., Molski T. F., Becker E. L., Sha'afi R. I. Characterization of the membrane-associated GTPase activity: effects of chemotactic factors and toxins. J Cell Biochem. 1989 Feb;39(2):197–206. doi: 10.1002/jcb.240390211. [DOI] [PubMed] [Google Scholar]
- Philips M. R., Abramson S. B., Kolasinski S. L., Haines K. A., Weissmann G., Rosenfeld M. G. Low molecular weight GTP-binding proteins in human neutrophil granule membranes. J Biol Chem. 1991 Jan 15;266(2):1289–1298. [PubMed] [Google Scholar]
- Postlethwaite A. E., Keski-Oja J., Moses H. L., Kang A. H. Stimulation of the chemotactic migration of human fibroblasts by transforming growth factor beta. J Exp Med. 1987 Jan 1;165(1):251–256. doi: 10.1084/jem.165.1.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts A. B., Anzano M. A., Lamb L. C., Smith J. M., Sporn M. B. New class of transforming growth factors potentiated by epidermal growth factor: isolation from non-neoplastic tissues. Proc Natl Acad Sci U S A. 1981 Sep;78(9):5339–5343. doi: 10.1073/pnas.78.9.5339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts A. B., Sporn M. B. Transforming growth factor beta. Adv Cancer Res. 1988;51:107–145. [PubMed] [Google Scholar]
- Rose F. R., Hirschhorn R., Weissmann G., Cronstein B. N. Adenosine promotes neutrophil chemotaxis. J Exp Med. 1988 Mar 1;167(3):1186–1194. doi: 10.1084/jem.167.3.1186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossi P., Karsenty G., Roberts A. B., Roche N. S., Sporn M. B., de Crombrugghe B. A nuclear factor 1 binding site mediates the transcriptional activation of a type I collagen promoter by transforming growth factor-beta. Cell. 1988 Feb 12;52(3):405–414. doi: 10.1016/s0092-8674(88)80033-3. [DOI] [PubMed] [Google Scholar]
- Sklar L. A., Hyslop P. A., Oades Z. G., Omann G. M., Jesaitis A. J., Painter R. G., Cochrane C. G. Signal transduction and ligand-receptor dynamics in the human neutrophil. Transient responses and occupancy-response relations at the formyl peptide receptor. J Biol Chem. 1985 Sep 25;260(21):11461–11467. [PubMed] [Google Scholar]
- Sklar L. A., Oades Z. G., Jesaitis A. J., Painter R. G., Cochrane C. G. Fluoresceinated chemotactic peptide and high-affinity antifluorescein antibody as a probe of the temporal characteristics of neutrophil stimulation. Proc Natl Acad Sci U S A. 1981 Dec;78(12):7540–7544. doi: 10.1073/pnas.78.12.7540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahl S. M., Hunt D. A., Wakefield L. M., McCartney-Francis N., Wahl L. M., Roberts A. B., Sporn M. B. Transforming growth factor type beta induces monocyte chemotaxis and growth factor production. Proc Natl Acad Sci U S A. 1987 Aug;84(16):5788–5792. doi: 10.1073/pnas.84.16.5788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakefield L. M., Smith D. M., Masui T., Harris C. C., Sporn M. B. Distribution and modulation of the cellular receptor for transforming growth factor-beta. J Cell Biol. 1987 Aug;105(2):965–975. doi: 10.1083/jcb.105.2.965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilde M. W., Carlson K. E., Manning D. R., Zigmond S. H. Chemoattractant-stimulated GTPase activity is decreased on membranes from polymorphonuclear leukocytes incubated in chemoattractant. J Biol Chem. 1989 Jan 5;264(1):190–196. [PubMed] [Google Scholar]
- Zigmond S. H., Slonczewski J. L., Wilde M. W., Carson M. Polymorphonuclear leukocyte locomotion is insensitive to lowered cytoplasmic calcium levels. Cell Motil Cytoskeleton. 1988;9(2):184–189. doi: 10.1002/cm.970090210. [DOI] [PubMed] [Google Scholar]