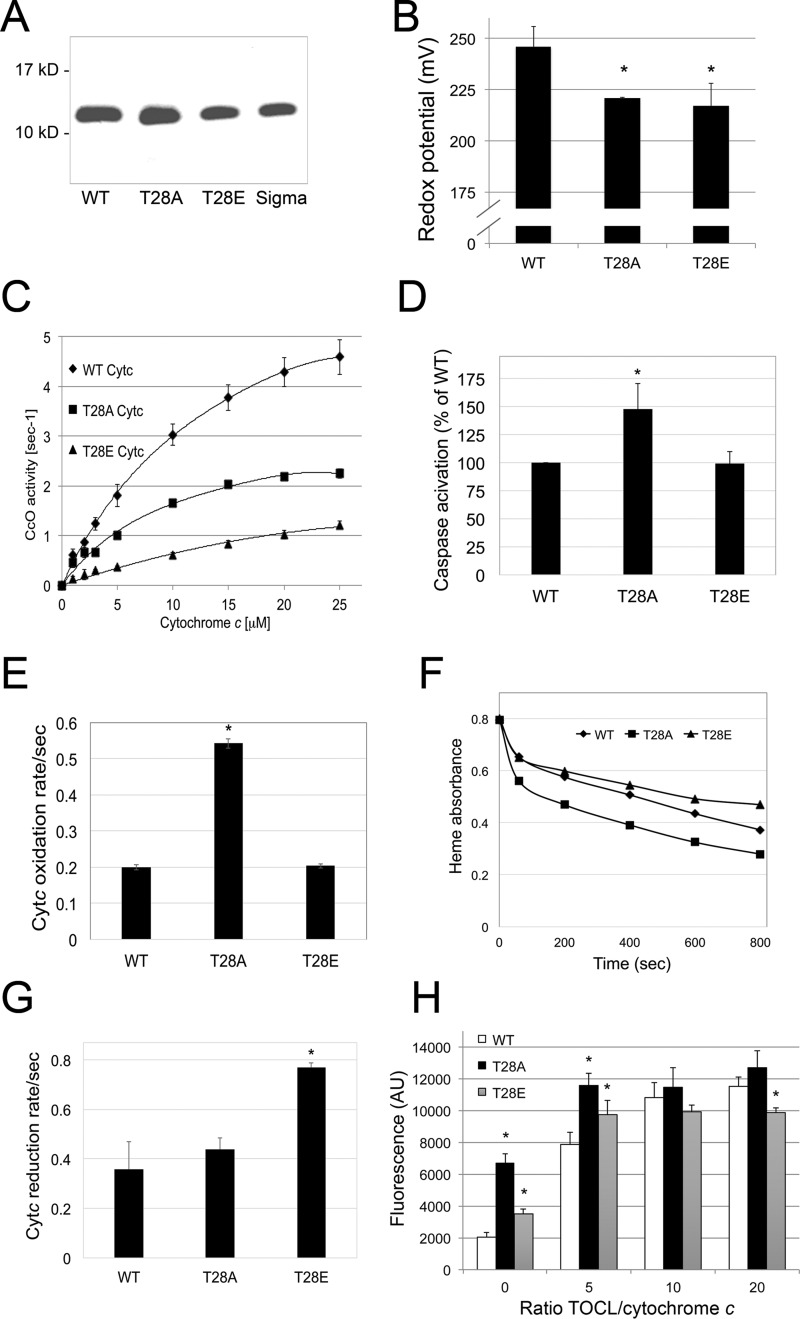
FIGURE 2.
Phosphomimetic T28E Cytc shows unique features in vitro. A, Coomassie gel of bacterially overexpressed and isolated WT, T28E (T28E), and T28A (T28A) Cytc indicates that the proteins were purified to homogeneity (Sigma; commercially available bovine Cytc as an additional control). B, redox potential is reduced in the T28E and T28A mutants compared with WT; means ± S.D. (error bars) are reported; *, p < 0.05. C, O2 consumption rates of cow cytochrome c oxidase in the reaction with Cytc were 73 and 51% reduced for T28E and T28A Cytc compared with WT. D, in vitro caspase-3 activity is unaltered with T28E Cytc, whereas it is increased with the T28A mutant. E, oxidation rate of WT and T28E Cytc in the presence of H2O2 is similar, whereas the T28A mutant shows increased rates. F, compared with WT, loss of the heme group by excess H2O2 is decreased and increased for T28E and T28A Cytc, respectively. Shown are representative heme destruction curves. G, the T28E Cytc reduction rate in the presence of ascorbate is increased compared with WT and T28A Cytc. H, T28E Cytc-mediated cardiolipin oxidation is decreased at highest TOCL/Cytc ratios compared with WT and T28A Cytc. AU, arbitrary units.