Abstract
Aims
Hypertension is highly prevalent in patients with aortic stenosis (AS) and is associated with worse outcomes. The current prospective study assessed the impact of systolic hypertension (SHPT) on the progression of aortic valve calcification (AVC) measured by multidetector computed tomography (MDCT) in patients with AS.
Methods and results
The present analysis includes the first series of 101 patients with AS prospectively recruited in the PROGRESSA study. Patients underwent comprehensive Doppler echocardiography and MDCT exams at baseline and after 2-year follow-up. AVC and coronary artery calcification (CAC) were measured using the Agatston method. Patients with SHPT at baseline (i.e. systolic blood pressure ≥140 mmHg; n = 37, 37%) had faster 2-year AVC progression compared with those without SHPT (i.e. systolic blood pressure <140 mmHg) (AVC median [25th percentile–75th percentile]: +370 [126–824] vs. +157 [58–303] AU; P = 0.007, respectively). Similar results were obtained with the analysis of AVC progression divided by the cross-sectional area of the aortic annulus (AVCdensity: +96 [34–218] vs. +45 [14–82] AU/cm2, P = 0.01, respectively). In multivariable analysis, SHPT remained significantly associated with faster progression of AVC or AVCdensity (all P = 0.001). There was no significant difference between groups with respect to progression of CAC (+39 [3–199] vs. +41 [0–156] AU, P = 0.88).
Conclusion
This prospective study shows for the first time that SHPT is associated with faster AVC progression but not with CAC progression in AS patients. These findings provide further support for the elaboration of randomized clinical trials to assess the efficacy of antihypertensive medication to slow the stenosis progression in patients with AS.
Keywords: aortic stenosis, calcific aortic valve disease, hypertension, Doppler echocardiography, multidetector computed tomography
Introduction
Calcific aortic stenosis (AS), third most common cardiovascular disease in Western countries, is characterized by a progressive calcification of the aortic valve leading to obstruction of LV outflow.1 Several epidemiological studies have reported a strong association between systemic arterial hypertension and the presence of calcific aortic valve disease.2,3 Furthermore, hypertension is highly prevalent (30–80%) and is associated with worse outcomes in patients with calcific AS.4–6 Systolic hypertension (SHPT) related to reduced arterial compliance is by far the most frequent form of hypertension in calcific AS population.5,6 In a cross-sectional study, Iwata et al.7 reported that higher ambulatory blood pressure is associated with higher prevalence of aortic valve calcification (AVC) as determined by echocardiography. A post-hoc analysis of the SEAS trial by Rieck et al.6 reported that hypertension is associated with a two-fold increased risk of mortality in asymptomatic patients with mild or moderate AS. In a retrospective study by Capoulade et al.,8 hypertension was associated with faster haemodynamic stenosis progression and higher mortality in patients with AS. Taking together, these findings support the hypothesis that hypertension may accelerate AS progression.
AVC is the main culprit lesion in calcific AS, and multidetector computed tomography (MDCT) has been shown to be accurate and reproducible to quantitatively determine AVC.9 Recent studies reported a strong relationship between AVC measured by MDCT and the haemodynamic stenosis severity and progression as well as the occurrence of adverse events in patients with AS.10,11 The objective of this prospective study was to examine the association between SHPT and AVC progression assessed by MDCT in patients with AS.
Methods
Patient population
The purpose and design of the PROGRESSA study were previously described12 and were detailed in the Supplementary data online. Briefly, patients with mild or moderate AS were prospectively recruited and underwent, at study entry, comprehensive Doppler echocardiography and MDCT exams within a time interval ≤3 months. Doppler echocardiography and MDCT were repeated at 2-year follow-up to measure the haemodynamic and anatomic (i.e. AVC) progression of AS, respectively. Among the 274 patients recruited in PROGRESSA between April 2005 and November 2014, 101 patients had a 2-year MDCT follow-up and were included in the present study (see Supplementary data online, Figure S1). The study was approved by the Ethics Committee of the Quebec Heart and Lung Institute, and all patients signed a written informed consent at the time of inclusion.
Clinical and medication data were detailed in the Supplementary dataonline. Laboratory data were previously described.12 Doppler echocardiographic data were described in the Supplementary data online.
Multidetector computed tomography data
MDCT were performed using a dual-source MDCT scanner (Somatom Definition, Siemens). MDCT acquisition and analyses were done by experienced technicians and cardiologists blinded to the clinical, laboratory, and Doppler echocardiographic data.
The protocol for the acquisition and interpretation of MDCT scans was previously described.9 Briefly, image analysis was performed off-line on dedicated workstations (Aquarius iNtuition, TeraRecon Inc.) for the measurements of AVC and coronary artery calcification (CAC) using the Agatston method, and results were expressed in arbitrary units (AU).13 As recently described, to account for interindividual variability in body size, we calculated the AVCdensity by dividing AVC score by the cross-sectional area of the aortic annulus measured by echocardiography.9 Total radiation exposure related to this study was <4 mSV.
Among the 101 patients included in this study, CAC score was not available for 11 patients (11%) due to image artifacts. The remaining 90 patients (89%) were included in the sub-analysis of CAC progression.
Study end points
The primary end point of this study was the progression of AVC and AVCdensity progression. The secondary end points were the progression of CAC (n = 90) and haemodynamic progression of AS as determined by Doppler echocardiography (n = 101). Progression rates of AVC, AVCdensity, CAC, and peak aortic jet velocity (Vpeak) were calculated by the difference between 2-year and baseline measurements. We examined the association between SHPT [systolic blood pressure (SBP) ≥140 mmHg at the time of baseline echocardiographic exam] on the primary and secondary end points. We also assessed the effect of the following parameters of arterial haemodynamics on outcomes: previous diagnosis of hypertension, as well as baseline diastolic blood pressure (DBP), pulse pressure (PP), systemic arterial compliance (SAC), and systemic vascular resistance (SVR).
Statistical analysis
Continuous data were expressed as mean ± SD and tested for the normality of distribution and homogeneity of variances using Shapiro–Wilk test and Levene tests, respectively. AVC, AVCdensity, and CAC at baseline and 2-year follow-up were not normally distributed and thus were presented as median [25th percentile–75th percentile] and were square-root transformed to normalize their distribution. Progression of AVC, AVCdensity, and CAC was not normally distributed despite data transformation and were presented as median [25th percentile–75th percentile]. Comparison of continuous variables between patients with SHPT and those without SHPT (SBP <140 mmHg) were performed using Student's t-test for normally distributed variables and Wilcoxon–Mann–Whitney for non-normally distributed variables. Kruskal–Wallis test followed by the Dunn's post-hoc test was used to compare non-normally distributed variables between more than two groups. Categorical data were expressed as percentage and compared with the χ2 test or Fisher's exact test if one or more cells have an expected frequency of five or less. Correlations were determined using Pearson's correlation coefficients for normally distributed variables or Spearman's correlation coefficients for non-normally distributed variables.
Individual linear regression analyses were performed to determine the association of SBP, SHPT, or isolated SHPT (SBP ≥140 mmHg and DBP <90 mmHg) with AVC, AVCdensity, CAC, or Vpeak progression. We determined whether SBP, SHPT, or isolated SHPT were independently associated with faster progression of AVC and AVCdensity using a multivariable linear regression analysis adjusted for variables with a P-value of <0.10 in individual analysis (i.e. age, sex, dyslipidemia, antihypertensive medication, creatinine, baseline haemodynamic AS severity, and baseline square-root transformed AVC) and clinical relevant variables (i.e. diabetes and metabolic syndrome). To determine the independent predictors of CAC progression, the multivariable model was adjusted for variables with a P-value of <0.10 and traditional risk factors (i.e. age, sex, PP, dyslipidaemia, fasting glucose, creatinine, and baseline square-root transformed CAC). Results were presented as standardized regression coefficients ± standard error (β coeff. ± SE) and coefficient of partial determination (Partial r2). Statistical analyses were performed with Stata Software (V.11.0). A P-value of <0.05 was considered statistically significant.
Results
Baseline characteristics of the population
Baseline characteristics of the 101 patients included in this study are presented in Table 1. Thirty-seven patients (37%) had SHPT in which 34 (92% of patients with SHPT) presented isolated SHPT and only 3 (8% of patients with SHPT) had systolo-diastolic hypertension (SBP ≥140mmHg and DBP ≥90 mmHg).
Table 1.
Baseline characteristics of the study population according to the presence or absence of SHPT
| Variables | No systolic hypertension (n = 64) | Systolic hypertension (n = 37) | P-value |
|---|---|---|---|
| Clinical | |||
| Age, years | 63 ± 13 | 69 ± 10 | 0.02 |
| Male, % | 70 | 81 | 0.23 |
| Height, cm | 168 ± 10 | 167 ± 7 | 0.56 |
| Weight, kg | 78 ± 16 | 84 ± 17 | 0.10 |
| Body surface area, m2 | 1.88 ± 0.22 | 1.92 ± 0.19 | 0.30 |
| Body mass index, kg/m2 | 27 ± 4 | 30 ± 5 | 0.03 |
| Systolic blood pressure, mmHg | 124 ± 12 | 152 ± 10 | – |
| Diastolic blood pressure, mmHg | 72 ± 9 | 80 ± 8 | <0.0001 |
| Pulse pressure, mmHg | 52 ± 11 | 72 ± 10 | <0.0001 |
| Heart rate, bpm | 62 ± 10 | 62 ± 9 | 0.90 |
| Hypertension, % | 64 | 84 | 0.04 |
| Dyslipidaemia, % | 60 | 81 | 0.04 |
| Diabetes, % | 17 | 27 | 0.24 |
| Coronary artery disease, % | 31 | 49 | 0.08 |
| Metabolic syndrome, % | 19 | 58 | <0.0001 |
| Medication | |||
| Antihypertensive medication, % | 66 | 81 | 0.10 |
| β-blockers, % | 27 | 35 | 0.36 |
| ACE inhibitors, % | 31 | 32 | 0.90 |
| ARB, % | 23 | 35 | 0.21 |
| Diuretics, % | 23 | 35 | 0.21 |
| Calcium antagonists, % | 20 | 41 | 0.03 |
| Other antihypertensive, % | 5 | 5 | 0.87 |
| Statin, % | 58 | 76 | 0.07 |
| Antidiabetics, % | 16 | 27 | 0.17 |
| Laboratory data | |||
| LDL cholesterol, mmol/L | 2.40 ± 0.88 | 2.20 ± 0.77 | 0.25 |
| HDL cholesterol, mmol/L | 1.45 ± 0.37 | 1.38 ± 0.41 | 0.39 |
| Triglycerides, mmol/L | 1.19 ± 0.45 | 1.76 ± 1.01 | 0.004 |
| Fasting glucose, mmol/L | 5.5 ± 1.0 | 6.1 ± 1.3 | 0.0007 |
| Creatinine, µmol/L | 83 ± 24 | 84 ± 17 | 0.84 |
| Doppler echocardiographic data | |||
| Bicuspid aortic valve, % | 27 | 14 | 0.13 |
| Peak aortic jet velocity, m/s | 2.8 ± 0.5 | 2.8 ± 0.4 | 0.95 |
| Mean transvalvular gradient, mmHg | 18 ± 7 | 17 ± 7 | 0.54 |
| Indexed aortic valve area, cm2/m2 | 0.67 ± 0.15 | 0.69 ± 0.14 | 0.72 |
| LV outflow tract diameter, cm | 2.2 ± 0.2 | 2.2 ± 0.2 | 0.40 |
| Relative wall thickness ratio | 0.51 ± 0.07 | 0.51 ± 0.08 | 0.90 |
| LV mass index, g/m2 | 100 ± 18 | 109 ± 20 | 0.02 |
| LV ejection fraction, % | 65 ± 5 | 65 ± 6 | 0.85 |
| Valvulo-arterial impedance, mmHg/mL/m2 | 3.4 ± 0.6 | 3.9 ± 0.7 | <0.0001 |
| Systemic arterial compliance, mL/m2/mmHg | 0.84 ± 0.23 | 0.60 ± 0.12 | <0.0001 |
| Systemic arterial compliance ≤0.6 mL/m2/mmHg, % | 8 | 53 | <0.0001 |
| Systemic vascular resistance, dyn·s/cm5 | 1510 ± 299 | 1720 ± 360 | 0.003 |
| Systemic vascular resistance >2000 dyn·s/cm5, % | 6 | 24 | 0.01 |
| MDCT data | |||
| Aortic valve | |||
| Aortic valve calcification, AU | 595 [338–1212] | 665 [406–1096] | 0.77 |
| Aortic valve calcification density, AU/cm2 | 170 [100–317] | 156 [102–266] | 0.66 |
| Coronary arteries | |||
| Coronary artery calcification (n = 90), AU | 362 [0–1034] | 620 [177–1291] | 0.07 |
Values are mean ± SD, % or median [25th percentile–75th percentile].
ACE, angiotensin-converting enzyme; ARB, angiotensin receptor blockers; AU, arbitrary unit(s); HDL, high-density lipoprotein; LDL, low-density lipoprotein; LV, left ventricular; MDCT, multidetector computed tomography.
Patients with SHPT at baseline were older (69 ± 10 vs. 63 ± 13 years, P = 0.02), had larger BMI (30 ± 5 vs. 27 ± 4 kg/m2, P = 0.03), and higher prevalence of dyslipidaemia (81 vs. 60%, P = 0.04) and metabolic syndrome (58 vs. 19%, P < 0.0001) (Table 1). Prevalence of bicuspid aortic valve, baseline haemodynamic severity of AS and LVEF were similar between groups but patients with SHPT had higher LV mass index (109 ± 20 vs. 100 ± 18 g/m2, P = 0.02) and global LV haemodynamic load as defined by Zva (3.9 ± 0.7 vs. 3.4 ± 0.6 mmHg/mL/m2, P < 0.0001) (Table 1).
Baseline AVC and AVCdensity were similar between groups, but there was a trend toward higher CAC at baseline in patients with SHPT compared with those without SHPT (620 [177–1291] vs. 362 [0–1034] AU, P = 0.07) (Table 1).
Association between SHPT and progression of AVC and CAC
Supplementary data online, Table S1, shows the univariable associations between arterial haemodynamic parameters and the progression of AVC, AVCdensity, CAC, and Vpeak. SBP, SHPT, isolated SHPT, PP, and SAC were all significantly associated with AVC and AVCdensity progression. There was no significant association between arterial haemodynamic parameters and progression of CAC and Vpeak.
Patients with SHPT at baseline had faster AVC progression compared with those without SHPT (+370 [126–824] vs. +157 [58–303] AU, P = 0.007; Figure 1; Table 2). Results were consistent with AVCdensity progression (+96 [34–218] vs. +45 [14–82] AU/cm2, P = 0.01; Figure 1 and Table 2). Supplementary data online, Table S2, shows the individual analyses for each variable included in the multivariable analysis. After adjustment for age, sex, dyslipidaemia, diabetes, metabolic syndrome, antihypertensive medication, creatinine level, baseline Vpeak, and baseline AVC or AVCdensity, SBP expressed in continuous variable, SHPT, or isolated SHPT were significantly associated with AVC or AVCdensity progression (Table 2). Adjustment for baseline mean transvalvular gradient (MG) or indexed aortic valve area (AVAi) instead of baseline Vpeak provided similar results (all P < 0.05). The variance inflation factor was <5 for all variables entered in the model, suggested that the level of multicollinearity in the multivariable analysis was acceptable.
Figure 1.
Progression of aortic valve calcification and coronary artery calcification according to the presence or absence of systolic hypertension. Comparison of the progression of AVC (A), and AVC indexed to the cross-sectional area of the aortic annulus (AVCdensity) (B) and CAC (n = 90) (C) according to the presence or absence of systolic hypertension (SHPT) at baseline [systolic blood pressure (SBP) ≥140 vs. <140 mmHg]. The box shows the 25th to 75th percentiles, the median line on the box shows the median value, and the error bars the 10th and 90th percentiles; circles are outliers; the numbers of the top of the graph are median [25th percentile–75th percentile]. Panel D shows representative MDCT images of aortic valve calcification at baseline and 2-year follow-up in two patients: Patient no.1 had a SBP of 143 mmHg at baseline, whereas Patient no. 2 had a SBP of 109 mmHg. AU, arbitrary unit(s); AVC, aortic valve calcification.
Table 2.
Univariable and multivariable analyses of the association between SHPT and progression of aortic valve calcification
| Univariable analysis |
Multivariable analysis |
|||||
|---|---|---|---|---|---|---|
| β Coeff. ± SE | P-value | β Coeff. ± SE | Partial r2 | P-value | Total r2 | |
| AVC progression, AU | ||||||
| SBP, mmHg | 0.29 ± 2.53 | 0.004 | 0.34 ± 2.61 | 0.12 | 0.001 | 0.28 |
| SHPT | 0.27 ± 92.9 | 0.006 | 0.36 ± 97.9 | 0.12 | 0.001 | 0.28 |
| Isolated SHPT | 0.30 ± 93.9 | 0.002 | 0.38 ± 96.1 | 0.14 | <0.0001 | 0.30 |
| AVCdensity progression, AU/cm2 | ||||||
| SBP, mmHg | 0.31 ± 0.60 | 0.002 | 0.34 ± 0.63 | 0.11 | 0.001 | 0.26 |
| SHPT | 0.29 ± 21.9 | 0.004 | 0.36 ± 23.7 | 0.11 | 0.001 | 0.26 |
| Isolated SHPT | 0.32 ± 22.1 | 0.001 | 0.37 ± 23.1 | 0.13 | <0.0001 | 0.28 |
β coeff. are standardized regression coefficient; partial r2 are coefficient of partial determination; total r2 are the overall r2 of the multivariable model. The multivariable analysis is adjusted for age, sex, dyslipidaemia, diabetes, metabolic syndrome, antihypertensive medication, creatinine level, baseline AS severity (i.e. peak aortic jet velocity), and square root of baseline aortic valve calcification.
AVC, aortic valve calcification; AVCdensity, aortic valve calcification indexed to cross-sectional area of the aortic annulus; SBP, systolic blood pressure; other abbreviations as in Table 1.
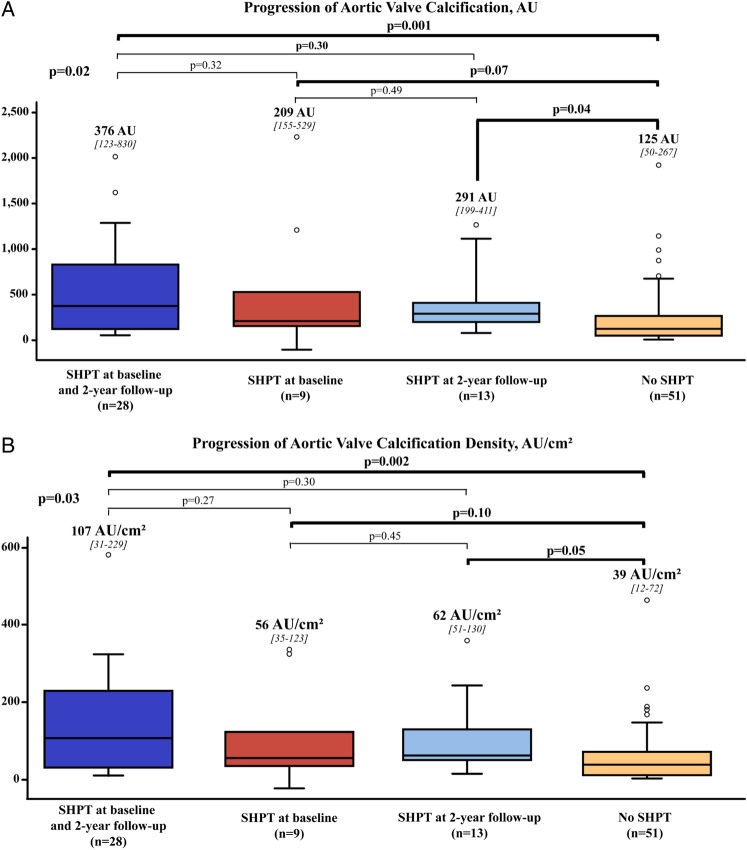
When analyzing the evolution of blood pressure at 2-year follow-up, we found that patients having SHPT both at baseline and at 2 years had two-fold faster progression of AVC or AVCdensity compared with those with normal blood pressure both at baseline and 2-year follow-up (all P < 0.01) (Figure 2). Interestingly, patients in the cross-over subgroups who had SHPT only at baseline or at 2-year follow-up had, respectively, a trend toward (P = 0.07) or significantly faster progression of AVC (P = 0.04) (Figure 2). Similar results were obtained with the progression of AVCdensity (Figure 2).
Figure 2.
Progression of aortic valve calcification according to the presence or absence of systolic hypertension at baseline and at 2-year follow-up. Comparison of the progression of AVC (A) and AVCdensity (B) according to the presence or absence of SHPT at baseline and 2-year follow-up. The four boxes represent from left to right: patients with SHPT both at baseline and follow-up; patients with SHPT at baseline but not at follow-up; patients with SHPT at follow-up but not at baseline; and patients with no SHPT at baseline and follow-up. Abbreviations as in Table 1.
Patients with SHPT had similar CAC progression compared with those without SHPT (+39 [3–199] vs. +41 [0–156] AU, P = 0.88; Figure 1). In individual analysis, baseline CAC score was significantly associated with CAC progression (β coeff. ± SE: 0.33 ± 1.34, P = 0.002). After adjustment for age, sex, PP, dyslipidaemia, fasting glucose, and creatinine levels, baseline CAC remained the unique independent predictor of CAC progression (β coeff. ± SE: 0.39 ± 1.80, P = 0.004).
Association between SHPT and haemodynamic progression
There was no significant difference in the progression of Vpeak from baseline to 2-year follow-up between patients with SHPT at baseline and those without SHPT (+21 [8–34] vs. +18 [2–40] cm/s, P = 0.72; see Supplementary data online, Figure S2). Similar results were obtained with the progression of MG and AVAi (MG: +3 [1–5] vs. +2 [0–6] mmHg, P = 0.43) (AVAi: −0.08 [−0.13 – −0.01] vs. −0.07 [−0.10–0] cm2/m2, P = 0.33). Nevertheless, there was a significant interaction between SHPT at baseline and AVC progression with respect to association with haemodynamic progression of AS (all interaction P < 0.05 for Vpeak, MG, and AVAi progression). In patients with SHPT, there was no association between AVC progression and Vpeak progression (P = 0.35), whereas in patients without SHPT, AVC progression was significantly correlated with Vpeak progression (P < 0.0001) (see Supplementary data online, Figure S3). Similar results were obtained with progression of MG and AVAi.
Discussion
The main findings of this study are that (i) SHPT is significantly associated with faster progression of AVC and AVCdensity but not of CAC progression. These findings suggest that the pathophysiological processes leading to valvular calcification may be different compared with those involved in CAC. (ii) Progression of AVC is associated with haemodynamic progression of AS in patients without SHPT but not in those with SHPT. This finding may be related to the fact that SHPT may interfere with the assessment of the haemodynamic severity of AS and therefore mask its progression during follow-up.
Association between SHPT and AVC progression
Calcific AS is an active and complex disorder, which involves a fibrocalcific remodelling and the osteogenic transition of valve interstitial cells (VICs).14 These processes ultimately lead to fibrosis and mineralization of the valve tissues.
In the present study, patients with SHPT had a two-fold faster progression of AVC. Elevated SBP may be a marker for the activation of the renin–angiotensin system (RAS). Previous studies suggest that angiotensin-II may promote fibrosis, remodelling, and calcification of aortic valve tissues.15,16 In mice, the administration of angiotensin-II resulted in significant thickening of the aortic leaflets. Likewise, in the hypercholesterolaemic rabbit, the administration of olmesartan, an angiotensin receptor blocker, prevented the mineralization/fibrosis of aortic valve.17,18 In human calcified aortic valves, angiotensin-converting enzyme (ACE) and chymase were expressed and colocalized with angiotensin-II.19 In pre-hypertensive patients operated for severe symptomatic AS, the circulating levels of angiotensin-II were associated with inflammation and fibrocalcific remodelling of the aortic valves.20 Moreover, the degrees of inflammation and remodelling were lower in calcified valves explanted from patients treated with angiotensin receptor blockers (ARBs).21
SHPT may increase the mechanical stress, and particularly the bending stress, on the valve leaflets during systole.22 Isolated SHPT with wide PP may thus be associated with more rapid and abrupt closing of the aortic valve, thus increasing the tensile stress during early diastole. Interestingly, a recent study including patients with aortic valve sclerosis (i.e. the preclinical stage of AS) demonstrated that higher diastolic ambulatory blood pressure was independently associated with AVC.7 Mechanical stress may cause endothelial damage and thereby enhance the infiltration of lipids and inflammatory cells within the valve.14 Also, elevated SBP may increase the mechanical strain on VICs, which activates cells to acquire a secretory phenotype.23 In a recent in vitro study, we found that human VICs overexpressed osteoblastic genes and produced mineralized microparticles when they were exposed to cyclic mechanical stretch similar to that encountered in hypertensive patients.24
SHPT is also associated with augmented amplitude and earlier reflection of arterial wave from the periphery which may disturb the blood flow pattern within the aortic sinuses and the shear stress on the aortic side of the valve during diastole.22
SHPT and progression of CAC
In patients with AS, AVC is the main factor responsible for the progression of the disease and the occurrence of events. However, in patients with coronary artery disease (CAD), the exact role of CAC in the occurrence of cardiac events is unclear. Although CAC is a marker of the overall coronary atherosclerosis burden, more calcium-dense atherosclerotic plaques are associated with lower risk of events than less calcium-dense plaques.25
Hypertension is a well-known risk factor for CAD, ischaemic cardiac events, and it has been associated with the incidence and progression of CAC.26 In the present AS population, there was no significant association between SHPT and CAC progression. However, consistent with previous data, baseline CAC was found as the unique and independent predictor of CAC progression.27 Although atherosclerosis and AS may often co-exist and share to some extent risk factors, our findings suggest that the mechanisms and clinical factors that determine the progression of mineralization may differ significantly between AS and CAD.
Clinical implications
Although several findings suggested that LDL cholesterol may have a role in the development and progression of AS,14 three randomized clinical trials did not demonstrate an effect of statin therapy on the progression rate of AS.28–30 This may be explained by the fact that the potential positive effects of statins at the valvular level (i.e. reduction of LDL cholesterol infiltration/oxidation and inflammation) may be counterbalanced by other negative effects (i.e. worsening of insulin resistance and small, dense LDL phenotype, increase in lipoprotein (a)). A recent study reported that, independent of their plaque regression effect, statins promote coronary atheroma calcification.31 Increasing calcification is potentially beneficial in the context of CAD as it may stabilize the plaque, but the same effect is likely detrimental in the context of AS where it promotes disease progression. Accordingly, we previously reported that in normocholesterolaemic patients with the metabolic syndrome that statin therapy was associated with a faster haemodynamic progression of AS.32
SHPT is highly prevalent in patients with calcific AS, and it has major impact on the outcomes of these patients.5,33–35 For a long time, vasodilatory therapy was perceived contraindicated in patients with AS, and particularly in those with severe AS, because of the potential risk of life-threatening hypotension in the setting of a fixed cardiac output. However, in the past decades, several studies have reported that therapies targeting the RAS are not only safe but are associated with significant improvement in outcomes.36,37
Retrospective studies suggest that antihypertensive therapy with ACE inhibitors or ARBs is associated with a slower haemodynamic progression rate of the stenosis, improved LV function, and lower mortality in patients with AS.8,38
Although further studies are needed to determine whether SHPT is a causal factor or simply a marker of calcific aortic valve disease process (Figure 1D), the results of the present prospective study provide further support to the realization of trials to test the efficacy of antihypertensive therapy for reducing disease progression in AS patients. Several recent studies8,18,21 suggest that ARBs may be the most promising medication for this purpose. The findings of this study also emphasize the importance of systematic screening of SHPT in patients with AS. AS patients with concomitant SHPT are at higher risk for rapid disease progression and should thus receive a closer clinical and echocardiographic follow-up.
This study also reveals that Doppler echocardiographic parameters of stenosis haemodynamic severity may not accurately reflect the progression of the culprit lesion (i.e. AVC) in the AS patients with concomitant SHPT. To this regard, several studies have shown that hypertension may interfere with the assessment of AS haemodynamic severity by echocardiography or cardiac catheterization.5,33–35 Hypertension may indeed yield to underestimation of stenosis severity and of its haemodynamic progression rate during follow-up. Hence, in AS patients with concomitant SHPT, echocardiographic assessment of haemodynamic severity should ideally be performed once blood pressure is normalized as recommended in the guidelines.39 Alternatively, disease progression can be measured in these patients with the use of AVC, a parameter that can be accurately measured by MDCT and that is not dependent of blood pressure and flow.
Limitations
In this study, blood pressure was measured by a trained nurse at the end of the echocardiographic exam, but we did not perform 24-h ambulatory recording, and there was no regular blood pressure follow-up during the 2-year follow-up. It is thus possible that we underestimated the prevalence of SHPT in this series.
This study demonstrated that SBP, SHPT, or isolated SHPT were significantly associated with faster progression of AVC as well as AVCdensity. However, the population size of this study may have limited our ability to detect weak but significant association with other factors. Also, the limited sample size did not allow for the analysis of the effect of the different types and dosage of antihypertensive medications on AVC progression. Nonetheless, SHPT remained independently associated with AVC progression after comprehensive adjustment that included antihypertensive medication.
Conclusion
This prospective study reports for the first time that SHPT is independently associated with faster progression of AVC but not with progression of CAC in patients with AS. These findings suggest that pathological processes involved in valvular vs. coronary artery mineralization may be different. Furthermore, these findings provide further support for the elaboration of randomized trials to test the efficacy of antihypertensive medication to slow the stenosis progression in patients with AS.
Supplementary data
Supplementary data are available at European Heart Journal—Cardiovascular Imaging online.
Funding
This work was supported by grants MOP-114997, MOP-2455048, and FDN-143225 from Canadian Institutes of Health Research (CIHR), Ottawa, Ontario, Canada and a grant from the Foundation of the Quebec Heart and Lung Institute. R.C. is supported by a post-doctoral fellowship grant from CIHR. E.L., M.A., and P.M. are research scholars from the Fonds de recherche Québec—Santé (FRQS), Montreal, Québec, Canada. J.-P.D. is the scientific director of the International Chair on Cardiometabolic Risk based at Université Laval. P.P. holds the Canada Research Chair in Valvular Heart Diseases from CIHR, Ottawa, Ontario, Canada.
Supplementary Material
Acknowledgements
We thank Isabelle Fortin, Karine Bibeau, Jocelyn Beauchemin, Céline Boutin, Louise Marois, Martine Poulin, Madeleine Dumont, Martine Parent, and Martine Fleury for their help in data collection and management.
Conflict of interest: J.-P.D. has served as a speaker for Abbott Laboratories, AstraZeneca, Solvay Pharma, GlaxoSmithKline, and Pfizer Canada, Inc.; has received research funding from Eli Lilly Canada; and has served on the advisory boards of Novartis, Theratechnologies, Torrent Pharmaceuticals Ltd., and Sanofi-Aventis. The other authors have reported no relationships relevant to the contents of this paper to disclose.
References
- 1.Nkomo VT, Gardin JM, Skelton TN, Gottdiener JS, Scott CG, Enriquez-Sarano M. Burden of valvular heart diseases: a population-based study. Lancet 2006;368:1005–11. [DOI] [PubMed] [Google Scholar]
- 2.Lindroos M, Kupari M, Valvanne J, Strandberg T, Heikkila J, Tilvis R. Factors associated with calcific aortic valve degeneration in the elderly. Eur Heart J 1994;15:865–70. [DOI] [PubMed] [Google Scholar]
- 3.Stewart BF, Siscovick D, Lind BK, Gardin JM, Gottdiener JS, Smith VE et al. . Clinical factors associated with calcific aortic valve disease. Cardiovascular Health Study. J Am Coll Cardiol 1997;29:630–4. [DOI] [PubMed] [Google Scholar]
- 4.Antonini-Canterin F, Huang G, Cervesato E, Faggiano P, Pavan D, Piazza R et al. . Symptomatic aortic stenosis: does systemic hypertension play an additional role? Hypertension 2003;41:1268–72. [DOI] [PubMed] [Google Scholar]
- 5.Briand M, Dumesnil JG, Kadem L, Tongue AG, Rieu R, Garcia D et al. . Reduced systemic arterial compliance impacts significantly on left ventricular afterload and function in aortic stenosis: implications for diagnosis and treatment. J Am Coll Cardiol 2005;46:291–8. [DOI] [PubMed] [Google Scholar]
- 6.Rieck AE, Cramariuc D, Boman K, Gohlke-Barwolf C, Staal EM, Lonnebakken MT et al. . Hypertension in aortic stenosis: implications for left ventricular structure and cardiovascular events. Hypertension 2012;60:90–7. [DOI] [PubMed] [Google Scholar]
- 7.Iwata S, Russo C, Jin Z, Schwartz JE, Homma S, Elkind MS et al. . Higher ambulatory blood pressure is associated with aortic valve calcification in the elderly: a population-based study. Hypertension 2013;61:55–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Capoulade R, Clavel MA, Mathieu P, Côté N, Dumesnil JG, Arsenault M et al. . Impact of hypertension and renin-angiotensin system inhibitors in aortic stenosis. Eur J Clin Invest 2013;43:1262–72. [DOI] [PubMed] [Google Scholar]
- 9.Clavel MA, Messika-Zeitoun D, Pibarot P, Aggarwal S, Malouf J, Araoz P et al. . The complex nature of discordant severe calcified aortic valve disease grading: new insights from combined Doppler-echocardiographic and computed tomographic study. J Am Coll Cardiol 2013;62:2329–38. [DOI] [PubMed] [Google Scholar]
- 10.Nguyen V, Cimadevilla C, Estellat C, Codogno I, Huart V, Benessiano J et al. . Haemodynamic and anatomic progression of aortic stenosis. Heart 2015;101:943–7. [DOI] [PubMed] [Google Scholar]
- 11.Clavel MA, Pibarot P, Messika-Zeitoun D, Capoulade R, Malouf J, Aggarval S et al. . Impact of aortic valve calcification, as measured by MDCT, on survival in patients with aortic stenosis: results of an international registry study. J Am Coll Cardiol 2014;64:1202–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Capoulade R, Mahmut A, Tastet L, Arsenault M, Bédard E, Dumesnil J et al. . Impact of plasma Lp-PLA2 activity on the progression of aortic stenosis. J Am Coll Cardiol Img 2015;8:26–33. [DOI] [PubMed] [Google Scholar]
- 13.Agatston AS, Janowitz WR, Hildner FJ, Zusmer NR, Viamonte M Jr, Detrano R. Quantification of coronary artery calcium using ultrafast computed tomography. J Am Coll Cardiol 1990;15:827–32. [DOI] [PubMed] [Google Scholar]
- 14.Rajamannan NM, Evans FJ, Aikawa E, Grande-Allen KJ, Demer LL, Heistad DD et al. . Calcific aortic valve disease: not simply a degenerative process: a review and agenda for research from the national heart and lung and blood institute aortic stenosis working group * executive summary: calcific aortic valve disease - 2011 update. Circulation 2011;124:1783–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Helske S, Syvaranta S, Kupari M, Lappalainen J, Laine M, Lommi J et al. . Possible role for mast cell-derived cathepsin G in the adverse remodelling of stenotic aortic valves. Eur Heart J 2006;27:1495–504. [DOI] [PubMed] [Google Scholar]
- 16.Akat K, Borggrefe M, Kaden JJ. Aortic valve calcification: basic science to clinical practice. Heart 2009;95:616–23. [DOI] [PubMed] [Google Scholar]
- 17.Arishiro K, Hoshiga M, Negoro N, Jin D, Takai S, Miyazaki M et al. . Angiotensin receptor-1 blocker inhibits atherosclerotic changes and endothelial disruption of the aortic valve in hypercholesterolemic rabbits. J Am Coll Cardiol 2007;49:1482–9. [DOI] [PubMed] [Google Scholar]
- 18.Fujisaka T, Hoshiga M, Hotchi J, Takeda Y, Jin D, Takai S et al. . Angiotensin II promotes aortic valve thickening independent of elevated blood pressure in apolipoprotein-E deficient mice. Atherosclerosis 2013;226:82–7. [DOI] [PubMed] [Google Scholar]
- 19.Helske S, Lindstedt KA, Laine M, Mayranpaa M, Werkkala K, Lommi J et al. . Induction of local angiotensin II-producing systems in stenotic aortic valves. J Am Coll Cardiol 2004;44:1859–66. [DOI] [PubMed] [Google Scholar]
- 20.Côté N, Pibarot P, Pepin A, Fournier D, Audet A, Arsenault B et al. . Oxidized low-density lipoprotein, angiotensin II and increased waist cirumference are associated with valve inflammation in prehypertensive patients with aortic stenosis. Int J Cardiol 2009;145:444–9. [DOI] [PubMed] [Google Scholar]
- 21.Cote N, Couture C, Pibarot P, Despres JP, Mathieu P. Angiotensin receptor blockers are associated with a lower remodelling score of stenotic aortic valves. Eur J Clin Invest 2011;41:1172–9. [DOI] [PubMed] [Google Scholar]
- 22.Arjunon S, Rathan S, Jo H, Yoganathan AP. Aortic valve: mechanical environment and mechanobiology. Ann Biomed Eng 2013;41:1331–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Balachandran K, Sucosky P, Yoganathan AP. Hemodynamics and mechanobiology of aortic valve inflammation and calcification. Int J Inflam 2011;2011:263870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bouchareb R, Boulanger MC, Fournier D, Pibarot P, Messaddeq Y, Mathieu P. Mechanical strain induces the production of spheroid mineralized microparticles in the aortic valve through a RhoA/ROCK-dependent mechanism. J Mol Cell Cardiol 2014;67:49–59. [DOI] [PubMed] [Google Scholar]
- 25.Criqui MH, Denenberg JO, Ix JO, McClelland RL, Wassel CL, Rifkin DE et al. . Calcium density of coronary artery plaque and risk of incident cardiovascular events. JAMA 2013;311:271–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kronmal RA, McClelland RL, Detrano R, Shea S, Lima JA, Cushman M et al. . Risk factors for the progression of coronary artery calcification in asymptomatic subjects: results from the Multi-Ethnic Study of Atherosclerosis (MESA). Circulation 2007;115:2722–30. [DOI] [PubMed] [Google Scholar]
- 27.Min JK, Lin FY, Gidseg DS, Weinsaft JW, Berman DS, Shaw LJ et al. . Determinants of coronary calcium conversion among patients with a normal coronary calcium scan: what is the “warranty period” for remaining normal? J Am Coll Cardiol 2010;55:1110–7. [DOI] [PubMed] [Google Scholar]
- 28.Cowell SJ, Newby DE, Prescott RJ, Bloomfield P, Reid J, Northridge DB et al. . A randomized trial of intensive lipid-lowering therapy in calcific aortic stenosis. N Engl J Med 2005;352:2389–97. [DOI] [PubMed] [Google Scholar]
- 29.Rossebo AB, Pedersen TR, Boman K, Brudi P, Chambers JB, Egstrup K et al. . Intensive lipid lowering with simvastatin and ezetimibe in aortic stenosis. N Engl J Med 2008;359:1343–56. [DOI] [PubMed] [Google Scholar]
- 30.Chan KL, Teo K, Dumesnil JG, Ni A, Tam J. Effect of lipid lowering with rosuvastatin on progression of aortic stenosis. Results of the aortic stenosis progression observation: Measuring effects of rosuvastatin (ASTRONOMER) trial. Circulation 2010;121:306–14. [DOI] [PubMed] [Google Scholar]
- 31.Puri R, Nicholls SJ, Shao M, Kataoka Y, Uno K, Kapadia SR et al. . Impact of statins on serial coronary calcification during atheroma progression and regression. J Am Coll Cardiol 2015;65:1273–82. [DOI] [PubMed] [Google Scholar]
- 32.Capoulade R, Clavel MA, Dumesnil JG, Chan KL, Teo KK, Tam JW et al. . Impact of metabolic syndrome on progression of aortic stenosis: influence of age and statin therapy. J Am Coll Cardiol 2012;60:216–23. [DOI] [PubMed] [Google Scholar]
- 33.Kadem L, Dumesnil JG, Rieu R, Durand LG, Garcia D, Pibarot P. Impact of systemic hypertension on the assessment of aortic stenosis. Heart 2005;91:354–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kadem L, Garcia D, Durand LG, Rieu R, Dumesnil JG, Pibarot P. Value and limitations of the peak-to-peak gradient for the evaluation of aortic stenosis. J Heart Valve Dis 2006;15:609–16. [PubMed] [Google Scholar]
- 35.Burwash IG, Lortie M, Pibarot P, de Kemp RA, Graf S, Mundigler G et al. . Myocardial blood flow in patients with low flow, low gradient aortic stenosis: differences between true and pseudo-severe aortic stenosis. Results from the multicenter TOPAS (Truly or Pseudo-Severe Aortic Stenosis) Study. Heart 2008;94:1627–33. [DOI] [PubMed] [Google Scholar]
- 36.Nadir MA, Wei L, Elder DH, Libianto R, Lim TK, Pauriah M et al. . Impact of renin-angiotensin system blockade therapy on outcome in aortic stenosis. J Am Coll Cardiol 2011;58:570–6. [DOI] [PubMed] [Google Scholar]
- 37.Bull S, Loudon M, Francis JM, Joseph J, Gerry S, Karamitsos TD et al. . A prospective, double-blind, randomized controlled trial of the angiotensin-converting enzyme inhibitor Ramipril In Aortic Stenosis (RIAS trial). Eur Heart J Cardiovasc Imaging 2015;16:834–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Goel SS, Aksoy O, Gupta S, Houghtaling PL, Tuzcu EM, Marwick T et al. . Renin-angiotensin system blockade therapy after surgical aortic valve replacement for severe aortic stenosis: a cohort study. Ann Intern Med 2014;161:699–710. [DOI] [PubMed] [Google Scholar]
- 39.Baumgartner H, Hung J, Bermejo J, Chambers JB, Evangelista A, Griffin BP et al. . Echocardiographic assessment of valve stenosis: EAE/ASE recommendations for clinical practice. J Am Soc Echocardiogr 2009;22:1–23. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.