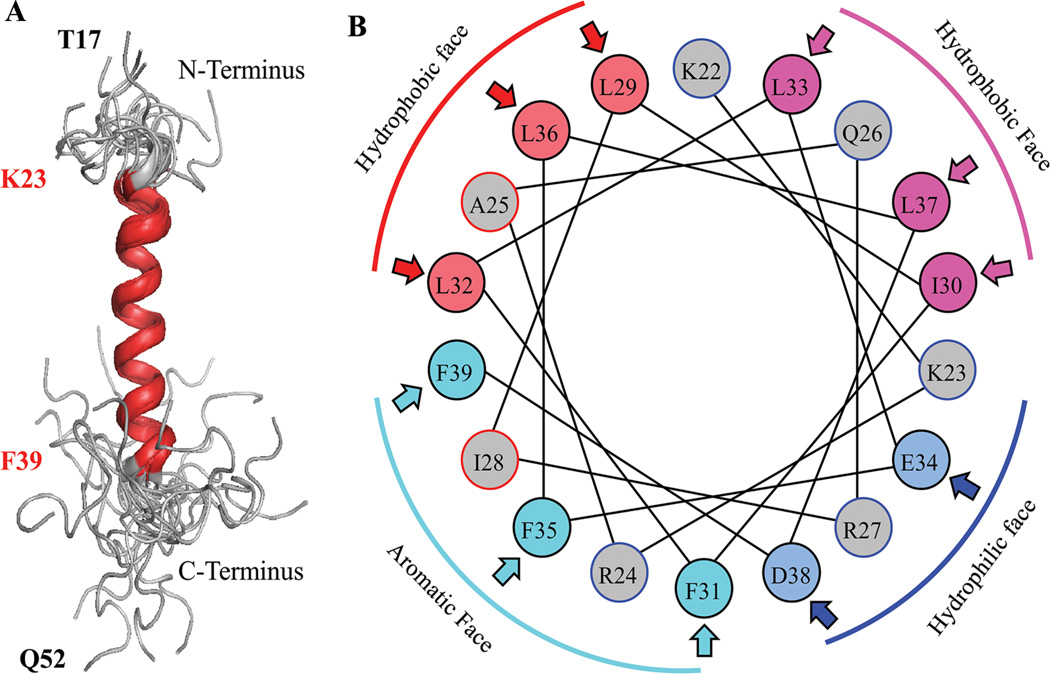
Fig. 5.
A: NMR structure of the agnoprotein peptide was determined in the presence of 30% trifluoroethanol (TFE) (pH 3.0, temperature 313K by calculation using ARIA [Nilges, 1995]) and CNS (Brunger et al., 1998) Superimposition of the 17 energetically most favorable structures of the peptide on the backbone atoms of the domain from Lys (K) 23 to Phe (F) 39 of the averaged structure. The a-helix region is designated in red and unstructured regions from residues Thr (T) 17 to Lys (K) 22 and Cys (C) 40 to Gln (Q) 52, are designated in gray. B: Helical wheel representation of the a-helix (Lys23 to Phe39) of the agnoprotein peptide. Hydrophilic and hydrophobic faces are indicated. Hydrophobic residues occupy approximately 75% of the surface area of the wheel while hydrophilic residues form a narrow surface.