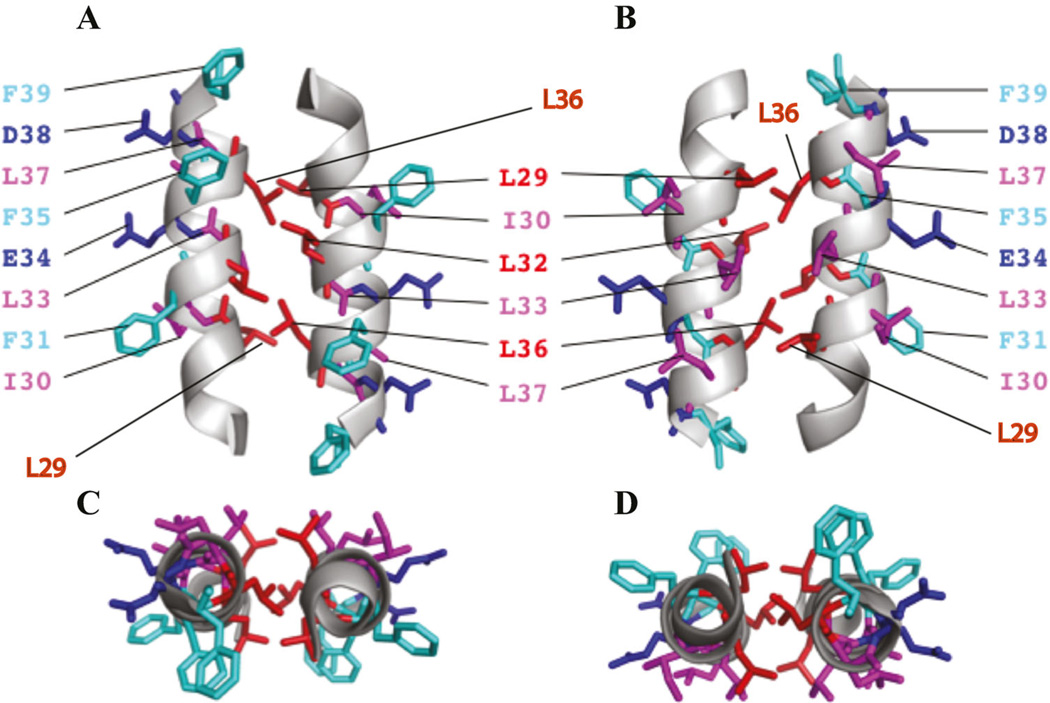
Fig. 6.
(A and B) A theoretical model of dimeric agnoprotein structure. The energetically most favorable structure of agnoprotein calculated with X-PLOR is shown with the backbone in a ribbon representation (Brunger, 1992; Brunger et al., 1998). The side chains of the aromatic and hydrophobic residues are shown in stick representations. The structure is characterized by two antiparallel a-helical domains from residues Lys23 to Phe39 with an interface composed of residues, Leu29, Leu32, and Leu36 (red). Residues Ile30, Leu33, and Leu37 (pink) are located at the opposite side of the aromatic residues Phe31, Phe35, and Phe39 (green). The part B is the 180° rotation presentaion of the part A on the y-axis. (C and D) Parts C and D represent a view from the top side of parts A and B, respectively.