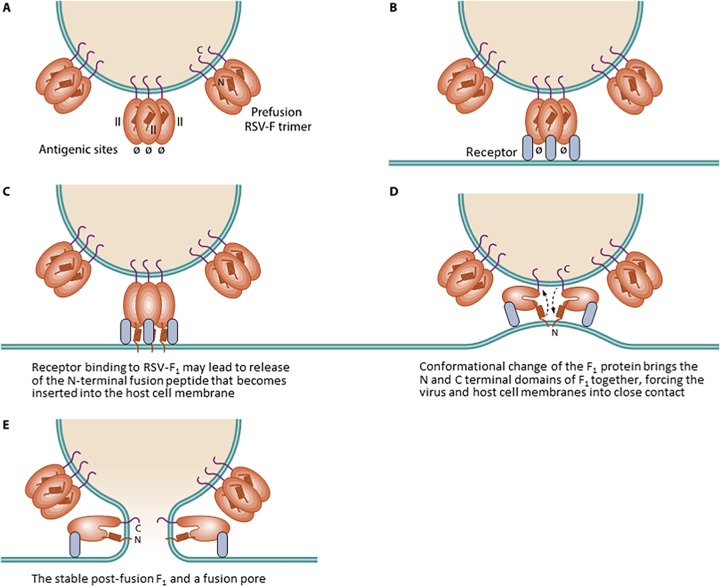
FIG 2.
Fusion process between the RSV envelope and cellular membrane. The RSV envelope has multiple protruding RSV-F fusion glycoproteins, anchored via transmembrane domains (A). In the prefusion state, RSV-F exists as a spring-loaded trimer with the major neutralization epitopes shown at the N-terminal region. The major antigenic site Ø exists only on the prefusion trimer and is lost after fusion. Interaction between the RSV-F trimer and a receptor may cause RSV-F to undergo a dramatic conformational shift (B), which leads to insertion of the fusion peptide into the host cell membrane (C) and forcing of the viral and host membranes into close contact (D). Although only two RSV-F monomers are depicted for simplicity, the combined force of multiple RSV-F conformational shifts is required to overcome the thermodynamic barrier of mixing membranes and establish a stable fusion pore for viral nucleocapsid delivery (E).