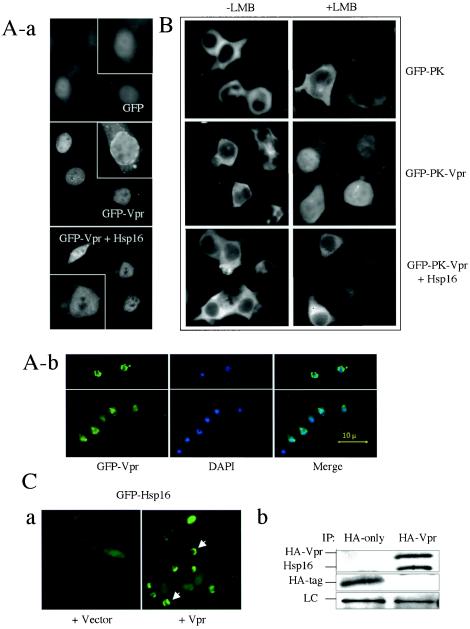
FIG.4.
Overexpression of hsp16 interferes with nuclear membrane localization and nuclear import of Vpr through direct protein-protein interaction. (A) Hsp16 diminishes nuclear membrane localization of Vpr in HeLa cells (a) and fission yeast (b). Note that Vpr localization on the nuclear membrane in HeLa cells can be distinguished as a clear ring surrounding the nucleus in the GFP-Vpr panel. However, this nuclear ring formed by GFP-Vpr was not clearly visible in GFP alone or GFP-Vpr+Hsp16 panels. Single-cell inserts in each panel show a magnified view of the presence or absence of nuclear membrane localization of Vpr. Nuclear membrane localization of Vpr was visualized by the expression of the GFP-Vpr fusion protein 24 h after transfection in HeLa cells. Cells containing the pYZ1N vector show Vpr localization on the nuclear membrane (panel b, top); cells containing pYZ1N-hsp16 show enhanced distribution of Vpr in the nucleus and cytoplasm (panel b, bottom). Nuclear membrane localization of Vpr was visualized 24 h after gene induction in fission yeast. DAPI staining was used to show nuclei. (B) Hsp16 blocks nuclear entry of Vpr in 293T cells. Nuclear import of Vpr was examined 24 h after transfection and 1 h after the addition of 400 nM LMB. (C) Potential interaction of Vpr with Hsp16. Panel a shows the effect of Vpr on subcellular localization of GFP-Hsp16 in strain Q1649. Note that expression of HIV-1 vpr changes the subcellular distribution of GFP-Hsp16 from evenly distributed (left) to a pattern that is similar to Vpr distribution, as indicated by arrows (right). Panel b shows the coimmunoprecipitation of Hsp16 with HA-tagged Vpr. Both anti-HA and anti-Hsp16 antibodies were used in Western blot analysis. LC, protein loading control.