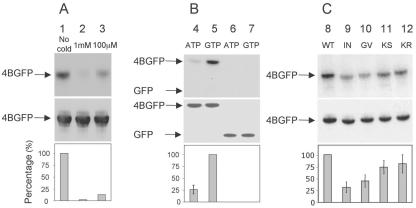
FIG. 3.
The NS4B NBM is specific for GTP and sensitive to genetic mutation. Each panel shows an autoradiograph (top), a Western blot analysis with an anti-GFP antibody (middle), and a graph quantifying nucleotide binding relative to wild-type control (bottom). (A) Binding of labeled GTP is progressively decreased in the presence of increasing concentrations of cold competitor nucleotide. Huh-7 cells were transfected with a plasmid encoding NS4B-GFP. Membrane preparations were incubated with 10 μM labeled GTP compound in the absence of cold GTPγS (lane 1) or presence of 1 mM (lane 2) or 100 μM (lane 3) competing cold GTPγS, followed by immunoprecipitation as in Fig. 2A. (B) NS4B-GFP binds ATP significantly less efficiently than GTP. Membrane preparations prepared from Huh-7 cells transfected with plasmids encoding NS4B-GFP (lanes 4 and 5) or GFP (lanes 6 and 7) were incubated with equal concentrations of labeled ATP (lanes 4 and 6) or GTP (lanes 5 and 7), followed by immunoprecipitation as in Fig. 2A. (C) Mutations within the NBM impair GTP binding. Huh-7 cells were transfected with plasmids encoding wild-type NS4B-GFP (lane 8) or NS4B-GFP with one of the following NBM mutations: Ile131Asn (lane 9), Gly129Val (lane 10), Lys135Ser (lane 11), or Lys135Arg (lane 12). As above, membrane fractions were incubated with labeled GTP, followed by immunoprecipitation. Experiments were repeated between two and four times. When present, any detectable binding of GTP to the 5A-GFP negative control protein was used for background subtraction purposes. Representative gels are shown. Mean values are plotted in the graphs, and error bars represent standard errors.