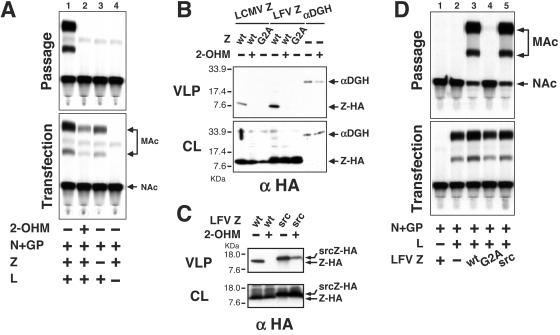
FIG. 2.
Inhibitors of myristoylation abolish VLP formation. (A) Treatment with 2-OHM eliminates VLP production. 293T cells were transfected with the indicated combination of plasmids in the presence (+) or absence (−) of 100 μM 2-OHM. Forty-eight hours later, SP were harvested and cell lysates were prepared. The presence of VLP in the SP was assessed as described for Fig. 1C. Aliquots from lysates of the transfected 293T (Transfection) and VLP-infected (Passage) cells were assayed for CAT activity. (B) Treatment with 2-OHM impairs self-budding activity of LCMV and LFV Z. 293T cells were transfected with the indicated plasmids, and 48 h later SP were saved and cell lysates were prepared. VLP-containing SP were subjected to centrifugation through a 20% sucrose cushion, and pellets resuspended in SDS-PAGE loading buffer. Aliquots of CL and VLP pellets (VLP) were analyzed by Western blot using an anti-HA antibody. (C) The first 10 amino acids of Src are sufficient to restore self-budding properties of G2A mutant Z proteins. 293T cells were transfected with the indicated plasmids in the presence (+) or absence (−) of 100 μM 2-OHM. Presence of Z protein in CL or medium SP was analyzed as in Fig. 2B. (D) Fusion of the myristoylation domain of Src to the N terminus of G2A mutant Z protein restored its function in VLP production. 293T cells were transfected with the indicated plasmids, and 48 h later SP were saved and CL were prepared. VLP-containing SP were used to infect fresh cells as described for Fig. 1C. Aliquots from lysates of the transfected 293T (Transfection) and VLP-infected (Passage) cells were assayed for CAT activity. NAc, nonacetylated chloramphenicol; MAc, monoacetylated chloramphenicol.